Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids - PubMed (original) (raw)
Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids
Dawn H Loh et al. Neuroendocrinology. 2008.
Abstract
Background/aims: Circadian control of behavior and physiology is a central characteristic of all living organisms. The master clock in mammals resides in the hypothalamus, where the suprachiasmatic nucleus (SCN) synchronizes daily rhythms. A variety of recent evidence indicates that the neuropeptide vasoactive intestinal peptide (VIP) is critical for normal functioning of the SCN. The aim of our study was to examine the possible role of VIP in driving circadian rhythms in the hypothalamic-pituitary-adrenal axis.
Methods: Circulating ACTH and corticosterone concentrations were determined by round-the-clock sampling under diurnal and circadian conditions. The responsive aspects of the hypothalamic-pituitary-adrenal axis were tested by application of acute stress by footshock and light.
Results: We demonstrate that the circadian rhythms in ACTH and corticosterone are lost in VIP-deficient mice. The ability of light to induce a corticosterone response was also compromised in the mutant mice, as was photic induction of Per1 in the adrenal glands. In contrast, the acute stress response was apparently unaltered by the loss of VIP.
Conclusion: Thus, our data demonstrate that VIP is essential for the circadian regulation of an otherwise intact hypothalamic-pituitary-adrenal axis.
Copyright 2008 S. Karger AG, Basel.
Figures
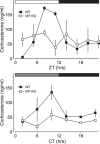
Figure 1
Concentration of corticosterone in serum over a 24 hour period in wild-type (WT) and _Vip_−/− (VIP KO) mice. Serum was sampled every 3 hours (_n_=3 per time point) under light-dark conditions (LD) and every 4 hours under constant darkness (DD). Circulating corticosterone concentration (ng/ml) was determined by ELISA. A. Serum corticosterone concentration under 12:12 LD conditions plotted against zeitgeber time (ZT). The WT peak in corticosterone at ZT8 was 30-fold higher than the trough at ZT2 and cycling conditions for WT concentrations were determined to be significant (P<0.001) by ANOVA. In contrast, VIP KO mice displayed no such rhythmicity in basal concentration of corticosterone. B. Corticosterone measurements plotted against circadian time (CT) in DD. Again, WT concentrations were determined to be significantly different by ANOVA (P<0.05) with a fourfold difference between peak and trough values at CT10 and CT2 respectively.
Figure 2
ACTH measurements from WT and VIP KO mice in LD plotted against ZT (A) and DD plotted against CT (B). Concentration of ACTH in the serum was determined by ELISA in 3 hour intervals in LD and 4 hour intervals in DD (_n_=2 or 3 for every time point). Circulating ACTH concentrations were found to be significantly rhythmic by ANOVA (P<0.01) for WT mice in LD and DD. The peak concentration of diurnal (LD) ACTH was determined to be at ZT11, and was 3-fold higher than at ZT2. Under DD, ACTH concentration peaked at CT12, where it was found to be 2-fold higher than CT4. In contrast, VIP KO mice did not display rhythmicity under either LD or DD, with circulating corticosterone concentrations remaining high throughout the 24-hour period.
Figure 3
Corticosterone response to acute stress by footshock in early night (ZT15). WT mice displayed a significant induction of corticosterone in response to acute stress (baseline: 33.63±16.07 ng/ml, _n_=5; vs. stress: 201.30±17.16 ng/ml, _n_=5; P<0.01). A similar corticosterone response to acute stress was also observed in VIP KO mice (baseline: 13.14±6.49 ng/ml, _n_=3; vs. stress 243.00±35.39 ng/ml, _n_=6; P<0.05). Statistical significances were determined by non-parametric Mann-Whitney t-tests.
Figure 4
Corticosterone induction by exposure to light in early night (ZT16). Serum corticosterone from untreated mice (_n_=4) and mice subjected to a light treatment (15μW/cm2, 30 minute duration, _n_=4) were determined by ELISA. WT mice respond to the light with a significance increase in circulating corticosterone concentration (283.60±28.84 ng/ml; P<0.05), but VIP KO mice failed to show a significant corticosterone response (33.42±17.79 ng/ml).
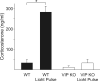
Figure 5
Photic induction of Per1 in the adrenal glands. 60 min after the start of the light treatment in early night (CT16; 15μW/cm2, 30 minute duration), adrenal glands were removed for RNA analysis by quantitative RT-PCR. Expression levels of Per1 were normalised to Hprt using the 2_−ΔΔCt_ method. WT mice showed a significant increase in relative Per1 expression (baseline: 3.29±0.43, _n_=4; vs. light pulse 14.37±1.27, _n_=4; P<0.001). VIP KO mice did not show a significant increase in Per1 expression (baseline: 5.76±0.59, _n_=4; vs. light pulse 5.93±1.01, _n_=4).
Similar articles
- Light entrainment of the SCN circadian clock and implications for personalized alterations of corticosterone rhythms in shift work and jet lag.
Li Y, Androulakis IP. Li Y, et al. Sci Rep. 2021 Sep 9;11(1):17929. doi: 10.1038/s41598-021-97019-7. Sci Rep. 2021. PMID: 34504149 Free PMC article. - Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus.
Kiessling S, Sollars PJ, Pickard GE. Kiessling S, et al. PLoS One. 2014 Mar 21;9(3):e92959. doi: 10.1371/journal.pone.0092959. eCollection 2014. PLoS One. 2014. PMID: 24658072 Free PMC article. - Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule.
Girotti M, Weinberg MS, Spencer RL. Girotti M, et al. Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E888-97. doi: 10.1152/ajpendo.90946.2008. Epub 2009 Feb 3. Am J Physiol Endocrinol Metab. 2009. PMID: 19190255 Free PMC article. - An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.
Harmar AJ. Harmar AJ. J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review. - Sex differences in circadian timing systems: implications for disease.
Bailey M, Silver R. Bailey M, et al. Front Neuroendocrinol. 2014 Jan;35(1):111-39. doi: 10.1016/j.yfrne.2013.11.003. Epub 2013 Nov 25. Front Neuroendocrinol. 2014. PMID: 24287074 Free PMC article. Review.
Cited by
- Vasoactive intestinal polypeptide immunoreactivity in the human cerebellum: qualitative and quantitative analyses.
Benagiano V, Flace P, Lorusso L, Rizzi A, Bosco L, Cagiano R, Ambrosi G. Benagiano V, et al. J Anat. 2009 Sep;215(3):256-66. doi: 10.1111/j.1469-7580.2009.01110.x. Epub 2009 Jun 22. J Anat. 2009. PMID: 19552726 Free PMC article. - Regulation of circadian and acute activity levels by the murine suprachiasmatic nuclei.
Houben T, Coomans CP, Meijer JH. Houben T, et al. PLoS One. 2014 Oct 8;9(10):e110172. doi: 10.1371/journal.pone.0110172. eCollection 2014. PLoS One. 2014. PMID: 25295522 Free PMC article. - Altered rhythm of adrenal clock genes, StAR and serum corticosterone in VIP receptor 2-deficient mice.
Fahrenkrug J, Georg B, Hannibal J, Jørgensen HL. Fahrenkrug J, et al. J Mol Neurosci. 2012 Nov;48(3):584-96. doi: 10.1007/s12031-012-9804-7. Epub 2012 May 24. J Mol Neurosci. 2012. PMID: 22622901 - Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus.
An S, Tsai C, Ronecker J, Bayly A, Herzog ED. An S, et al. J Comp Neurol. 2012 Aug 15;520(12):2730-41. doi: 10.1002/cne.23078. J Comp Neurol. 2012. PMID: 22684939 Free PMC article. - Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver.
Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pévet P, Buijs RM. Cailotto C, et al. PLoS One. 2009 May 21;4(5):e5650. doi: 10.1371/journal.pone.0005650. PLoS One. 2009. PMID: 19478857 Free PMC article.
References
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. - PubMed
- Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18:250–260. - PubMed
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. - PubMed
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. - PubMed
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in rat. Brain Research. 1972;42:201–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS-043169/NS/NINDS NIH HHS/United States
- R01 NS043169-05/NS/NINDS NIH HHS/United States
- MH-65497/MH/NIMH NIH HHS/United States
- R01 NS043169-04/NS/NINDS NIH HHS/United States
- R01 NS043169/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical