Interactions between APP secretases and inflammatory mediators - PubMed (original) (raw)
Review
Interactions between APP secretases and inflammatory mediators
Magdalena Sastre et al. J Neuroinflammation. 2008.
Abstract
There is now a large body of evidence linking inflammation to Alzheimer's disease (AD). This association manifests itself neuropathologically in the presence of activated microglia and astrocytes around neuritic plaques and increased levels of inflammatory mediators in the brains of AD patients. It is considered that amyloid-beta peptide (Abeta), which is derived from the processing of the longer amyloid precursor protein (APP), could be the most important stimulator of this response, and therefore determining the role of the different secretases involved in its generation is essential for a better understanding of the regulation of inflammation in AD. The finding that certain non-steroidal anti-inflammatory drugs (NSAIDs) can affect the processing of APP by inhibiting beta- and gamma-secretases, together with recent revelations that these enzymes may be regulated by inflammation, suggest that they could be an interesting target for anti-inflammatory drugs. In this review we will discuss some of these issues and the role of the secretases in inflammation, independent of their effect on Abeta formation.
Figures
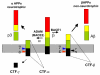
Figure 1
Proteolytic processing of APP. Proteolysis of APP by α-secretase or β-secretase leads to the secretion of αAPPs or βAPPs. Both secretases generate C-terminal fragments (CTF) of 10 kDa and 12 kDa respectively, which are inserted in the membrane (grey). These fragments can be cut by γ-secretase to release the peptides P3 and Aβ. Two further cleavage sites, termed ε and ζ, have been identified in the CTF.
Figure 2
Schematic representation of the interactions between inflammatory processes and APP processing. Aβ generation by BACE1 and γ-secretase induces an inflammatory response, which involves the activation of microglia and astrocytes and the release of pro-inflammatory cytokines. This inflammatory response could be enhanced by brain trauma or by PS1 mutations, probably also by increased Aβ production. Inflammatory cytokines have been involved in the aggregation of Aβ [121]. Moreover, cytokines can affect the expression of secretases and APP, influencing their transcription, translation and/or activation [122]. Non-steroidal anti-inflammatory drug treatment could reverse the effect of inflammation on BACE1 transcription, modulate the cleavage site of γ-secretase and decrease the secretion of cytokines and the number of microglia and astrocytes. On the other hand, α-secretase has been involved in the shedding of certain cytokines, potentiating their activity. See text for further details of individual interactions. Numbers on the diagram correspond to the appropriate references in the review.
Similar articles
- Activation of peroxisome proliferator-activated receptor α stimulates ADAM10-mediated proteolysis of APP.
Corbett GT, Gonzalez FJ, Pahan K. Corbett GT, et al. Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):8445-50. doi: 10.1073/pnas.1504890112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080426 Free PMC article. - Upregulation of APP, ADAM10 and ADAM17 in the denervated mouse dentate gyrus.
Del Turco D, Schlaudraff J, Bonin M, Deller T. Del Turco D, et al. PLoS One. 2014 Jan 3;9(1):e84962. doi: 10.1371/journal.pone.0084962. eCollection 2014. PLoS One. 2014. PMID: 24404197 Free PMC article. - The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer's disease.
Tan JZA, Gleeson PA. Tan JZA, et al. Biochim Biophys Acta Biomembr. 2019 Apr 1;1861(4):697-712. doi: 10.1016/j.bbamem.2018.11.013. Epub 2019 Jan 11. Biochim Biophys Acta Biomembr. 2019. PMID: 30639513 Review. - Transcriptional and translational regulation of BACE1 expression--implications for Alzheimer's disease.
Rossner S, Sastre M, Bourne K, Lichtenthaler SF. Rossner S, et al. Prog Neurobiol. 2006 Jun;79(2):95-111. doi: 10.1016/j.pneurobio.2006.06.001. Epub 2006 Aug 14. Prog Neurobiol. 2006. PMID: 16904810 Review.
Cited by
- TLR4 inhibitor TAK-242 attenuates the adverse neural effects of diet-induced obesity.
Moser VA, Uchoa MF, Pike CJ. Moser VA, et al. J Neuroinflammation. 2018 Nov 5;15(1):306. doi: 10.1186/s12974-018-1340-0. J Neuroinflammation. 2018. PMID: 30396359 Free PMC article. - Inhibition of IKKβ by celastrol and its analogues - an in silico and in vitro approach.
Veerappan K, Natarajan S, Ethiraj P, Vetrivel U, Samuel S. Veerappan K, et al. Pharm Biol. 2017 Dec;55(1):368-373. doi: 10.1080/13880209.2016.1241809. Pharm Biol. 2017. PMID: 27931154 Free PMC article. - Impact of Cytokines and Chemokines on Alzheimer's Disease Neuropathological Hallmarks.
Domingues C, da Cruz E Silva OAB, Henriques AG. Domingues C, et al. Curr Alzheimer Res. 2017;14(8):870-882. doi: 10.2174/1567205014666170317113606. Curr Alzheimer Res. 2017. PMID: 28317487 Free PMC article. Review. - ADAM-17: the enzyme that does it all.
Gooz M. Gooz M. Crit Rev Biochem Mol Biol. 2010 Apr;45(2):146-69. doi: 10.3109/10409231003628015. Crit Rev Biochem Mol Biol. 2010. PMID: 20184396 Free PMC article. Review. - CART mitigates oxidative stress and DNA damage in memory deficits of APP/PS1 mice via upregulating β‑amyloid metabolism‑associated enzymes.
Jiang H, Niu F, Zheng Y, Xu Y. Jiang H, et al. Mol Med Rep. 2021 Apr;23(4):280. doi: 10.3892/mmr.2021.11919. Epub 2021 Feb 19. Mol Med Rep. 2021. PMID: 33604684 Free PMC article.
References
- Alzheimer A. Ueber eine eigenartige Erkrankung der Hirnrinde. Centralblatt fur Nervenheilkunde un Psychiatrie. 1907;30:177–179.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous