Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages - PubMed (original) (raw)
Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages
Jason L Johnson et al. Arterioscler Thromb Vasc Biol. 2008 Sep.
Abstract
Objective: An excess of metalloproteinases (MMPs) over tissue inhibitors of metalloproteinases (TIMPs) may favor atherosclerotic plaque rupture. We compared TIMP levels in nonfoamy and foam-cell macrophages (FCM) generated in vivo.
Methods and results: In vivo generated rabbit FCM exhibited 84% reduced TIMP-3 protein compared to nonfoamy macrophages, and immunocytochemistry revealed a TIMP-3 negative subset (28%). Strikingly, only TIMP-3 negative FCM invaded a synthetic basement membrane, and invasion was inhibited by exogenous TIMP-3. TIMP-3 negative FCM also had increased proliferation and apoptosis rates compared to TIMP-3 positive cells, which were retarded by exogenous TIMP-3; this also reduced gelatinolytic activity. TIMP-3 negative FCM were found at the base of advanced rabbit plaques and in the rupture-prone shoulders of human plaques. To explain the actions of low TIMP-3 we observed a 26-fold increase in MT1-MMP (MMP-14) protein in FCM. Adding an MT1-MMP neutralizing antibody reduced foam-cell invasion, apoptosis, and gelatinolytic activity. Furthermore, MT1-MMP overexpressing and TIMP-3 negative FCM were found at the same locations in atherosclerotic plaques.
Conclusions: These results demonstrate that TIMP-3 is downregulated in a distinct subpopulation of FCM which have increased MMP-14. These cells are highly invasive and have increased proliferation and apoptosis, all properties expected to destabilise atherosclerotic plaques.
Figures
Figure 1
Effect of foam-cell macrophage formation and their subsequent migration on TIMP-3 protein expression. TIMP-3 expression in (A) macrophages, (B) foam-cell macrophages, and (C) foam-cell macrophages on matrigel plus exogenous TIMP-3 (arrows indicate TIMP-3 negative cells). TIMP-3 expression in (D) Nonmigrated foam-cells (black arrows) and (E) migrated foam-cells (white arrows). F, Migrated foam-cells are RAM11 positive (arrows). G, Nonimmune IgG on migrated foam-cell macrophages.
Figure 2
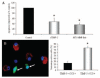
Effects of TIMP-3 expression and addition on foam-cell macrophage apoptosis. A, Exogenous TIMP-3 or a MT1-MMP neutralizing antibody inhibits foam-cell macrophage apoptosis (*P<0.05, n=3). B, Dual immunofluoresence highlights that loss of TIMP-3 expression (red) in foam-cell macrophages is associated with apoptosis as detected by cleaved caspase-3 (green). Quantification is summarized in the adjoining graph (*P<0.05, n=4).
Figure 3
Effect of exogenous TIMP-3 and MT1-MMP inhibition on foam-cell macrophage gelatinolytic activity. A, Exogenous TIMP-3 or a MT1-MMP neutralizing antibody inhibits foam-cell macrophage gelatinolytic activity (green), as indicated by white arrows. Quantification is summarized in the adjoining graph (*P<0.05, n=3). B and C, MT1-MMP expression of non-(arrows) and migrated (arrowheads) foam-cell macrophages from Matrigel-coated transwell inserts.
Figure 4
TIMP-3 localization in rabbit atherosclerotic plaques. Immunohistochemical labeling of early (A–C) and advanced (D–H) rabbit atherosclerotic plaques, for TIMP-3, macrophages (RAM11), or both. D, Nonimmune IgG control. H, Dual immunohistochemistry for macrophages (blue/green) and TIMP-3 (brown) in an advanced rabbit atherosclerotic plaque, demonstrating TIMP-3 positive macrophages (arrows) within the adventitia.
Figure 5
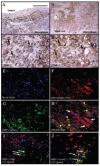
TIMP-3 localization in human advanced atherosclerotic plaques. Immunohistochemical labeling of advanced human carotid atherosclerotic plaques for macrophages (A and B) or TIMP-3 (C and D). Confocal microscopy for macrophages (F, red), TIMP-3 (G, green), and merged (H). Dual immunohistochemical labeling for cells undergoing apoptosis (green) and either TIMP-3 (red, I) or MT1-MMP (red, J).
Figure 6
MT1-MMP localization in rabbit atherosclerotic plaques. Immunohistochemical labeling of advanced rabbit atherosclerotic plaques, for macrophages (A and D), MT1-MMP (B and E), or TIMP-3 (C and F). Box in panels (A, B, and C) represents respective higher magnification in panels (D, E, and F). Arrows indicate immunopositive cells (brown). Dotted lines represent atherosclerotic plaque/medial boundary.
Similar articles
- Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability.
Johnson JL, Jenkins NP, Huang WC, Di Gregoli K, Sala-Newby GB, Scholtes VP, Moll FL, Pasterkamp G, Newby AC. Johnson JL, et al. Mediators Inflamm. 2014;2014:276457. doi: 10.1155/2014/276457. Epub 2014 Aug 24. Mediators Inflamm. 2014. PMID: 25301980 Free PMC article. - Differential inhibition of membrane type 3 (MT3)-matrix metalloproteinase (MMP) and MT1-MMP by tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3 rgulates pro-MMP-2 activation.
Zhao H, Bernardo MM, Osenkowski P, Sohail A, Pei D, Nagase H, Kashiwagi M, Soloway PD, DeClerck YA, Fridman R. Zhao H, et al. J Biol Chem. 2004 Mar 5;279(10):8592-601. doi: 10.1074/jbc.M308708200. Epub 2003 Dec 16. J Biol Chem. 2004. PMID: 14681236 - Gene expression levels of matrix metalloproteinases in human atherosclerotic plaques and evaluation of radiolabeled inhibitors as imaging agents for plaque vulnerability.
Müller A, Krämer SD, Meletta R, Beck K, Selivanova SV, Rancic Z, Kaufmann PA, Vos B, Meding J, Stellfeld T, Heinrich TK, Bauser M, Hütter J, Dinkelborg LM, Schibli R, Ametamey SM. Müller A, et al. Nucl Med Biol. 2014 Aug;41(7):562-9. doi: 10.1016/j.nucmedbio.2014.04.085. Epub 2014 Apr 21. Nucl Med Biol. 2014. PMID: 24853402 - Metalloproteinase production from macrophages - a perfect storm leading to atherosclerotic plaque rupture and myocardial infarction.
Newby AC. Newby AC. Exp Physiol. 2016 Nov 1;101(11):1327-1337. doi: 10.1113/EP085567. Epub 2016 May 5. Exp Physiol. 2016. PMID: 26969796 Review. - Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability.
Newby AC. Newby AC. Arterioscler Thromb Vasc Biol. 2008 Dec;28(12):2108-14. doi: 10.1161/ATVBAHA.108.173898. Epub 2008 Sep 4. Arterioscler Thromb Vasc Biol. 2008. PMID: 18772495 Review.
Cited by
- Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages.
Gill SE, Gharib SA, Bench EM, Sussman SW, Wang RT, Rims C, Birkland TP, Wang Y, Manicone AM, McGuire JK, Parks WC. Gill SE, et al. Am J Respir Cell Mol Biol. 2013 Nov;49(5):768-77. doi: 10.1165/rcmb.2012-0377OC. Am J Respir Cell Mol Biol. 2013. PMID: 23742180 Free PMC article. - Proprotein convertase subtilisin/kexin type 3 promotes adipose tissue-driven macrophage chemotaxis and is increased in obesity.
Kappert K, Meyborg H, Fritzsche J, Urban D, Krüger J, Wellnhofer E, Kintscher U, Fleck E, Stawowy P. Kappert K, et al. PLoS One. 2013 Aug 6;8(8):e70542. doi: 10.1371/journal.pone.0070542. Print 2013. PLoS One. 2013. PMID: 23936445 Free PMC article. - The role of matrix metalloproteinases in atherothrombosis.
Ketelhuth DF, Bäck M. Ketelhuth DF, et al. Curr Atheroscler Rep. 2011 Apr;13(2):162-9. doi: 10.1007/s11883-010-0159-7. Curr Atheroscler Rep. 2011. PMID: 21271310 Review. - Innate immunity and monocyte-macrophage activation in atherosclerosis.
Shalhoub J, Falck-Hansen MA, Davies AH, Monaco C. Shalhoub J, et al. J Inflamm (Lond). 2011 Apr 28;8:9. doi: 10.1186/1476-9255-8-9. J Inflamm (Lond). 2011. PMID: 21526997 Free PMC article. - Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB.
Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Huang WC, et al. PLoS One. 2012;7(8):e42507. doi: 10.1371/journal.pone.0042507. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880008 Free PMC article.
References
- Newby AC. Dual role of matrix metalloproteinases (matrixins) in neointima formation and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. - PubMed
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. - PubMed
- Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69:625–635. - PubMed
- Boyle JJ, Weissberg PL, Bennett MR. Human Macrophage-Induced Vascular Smooth Muscle Cell Apoptosis Requires NO Enhancement of Fas/Fas-L Interactions. Arterioscler Thromb Vasc Biol. 2002;22:1624–1630. - PubMed
- Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5:265–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous