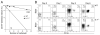IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression - PubMed (original) (raw)
IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression
Kazu Kikuchi et al. J Immunol. 2008.
Abstract
IL-7 plays a critical role in B cell fate decision by regulating early B cell factor (EBF) expression. However, it was not clear when IL-7 stimulation is necessary in hemato-/lymphopoiesis in adult mice. Here we show that pre-proB cells derived from IL-7-/- mice have lost B cell potential, despite up-regulation of EBF expression following IL-7 stimulation. Pre-proB cells from wild-type mice can give rise to proB cells in the absence of IL-7. In this case, EBF up-regulation during the transition from the pre-proB to proB stages occurs normally. In contrast, EBF expression by IL-7-/- pre-proB cells after IL-7 stimulation is approximately 20 times lower than wild-type pre-proB cells. In addition, only multipotent progenitors with higher levels of ectopic EBF can give rise to proB cells in the absence of IL-7. Therefore, the primary function of IL-7 before the pre-proB stage in B cell development is to maintain the EBF expression level above a certain threshold, which is necessary for pre-proB cells to further transit to the proB stage.
Figures
Figure 1. Purity of CLPs and pre-proB cells after sorting
CLPs (A) and pre-proB cells (B) were doubly sorted as Lin−IL-7Rα+Thy-1−Sca-1loc-Kitlo and B220+CD43+CD19−NK1.1−Ly-6C−, respectively as shown previously (9, 17). After sorting, we examined the purity of the population on FACS. Shown FACS plots are pre-gated on the PI− fraction to exclude dead cells. Since both Lin+ and PI+ cells were gated out in the same PE/Cy5 channel in CLP sorting, Lin expression is not shown in (A). After sorting CLPs and pre-proB cells twice, the purity of the populations is over 99%.
Figure 2. In vivo B cell potential of CLPs and pre-proB cells derived from IL-7−/− mice. B cell
potential of CLPs (A) and pre-proB cells (B) was examined by in vivo reconstitution assay. CLPs (2.0×103) and pre-proB cells (1.4×104) derived from WT or IL-7−/− mice (CD45.2+) were injected into sub-lethally irradiated RAG2−/− mice (CD45.1+). B cell readout was analyzed in the spleen of host mice at 2 weeks post injection. Representative data from three independent experiments were shown. The mean ± S.D. from a total of 5–6 reconstituted mice in each group was calculated and indicated in the FACS plots.
Figure 3. B cell potential of IL-7−/− pre-proB cells in in vitro stromal cell cultures.(A)
Pre-proB cells (1.0×104 cells/well) derived from WT (upper panels) and IL-7−/− mice (lower panels) were cultured on OP9 stromal cells in the presence of IL-7, SCF, and Flt3L for the period shown in the figures. Representative FACS plots from two independent experiments were shown. The mean ± S.D. from more than 6 samples from various time points was indicated in the FACS plots. CD19+ cells in the plots represent proB cells. (B) Apoptotic cells were examined by annexin V staining at 6 days after culture. Stromal cells were excluded by scatter gates. PI exclusion was not done in this assay. (C) The frequency of cells that can give rise to proB cells in the pre-proB population in WT and IL-7−/− pre-proB cells. Indicated numbers of pre-proB cells from either WT (open circle) or IL-7−/− pre-proB cells (closed circle) were cultured in 96 well plates as described in the legend for Figure 2A. Wells containing B220+CD19+ cells were counted as a positive well. We did not observe any CD19+ proB cells from IL-7−/− pre-proB cells even at 1.0×103 cells/well, where all wells seeded with WT pre-proB cells contained proB cells (the limiting number: 1 in 212). (D) Cell numbers in the culture of pre-proB cells. Cell numbers in the culture shown in Figure 2A were counted with a hemocytometer under the microscope. OP9 stromal cells were excluded from the counting based on the difference in cell size. The shaded area in the graph indicates the numbers below the input cell number (1×104). (E) Cell cycle status of pre-proB and proB cells in WT mice was analyzed on FACS.
Figure 4. IL-7R/Jak/Stat signaling pathway is intact in IL-7−/− pre-proB cells
(A) IL-7Rα and _γ_c expression were examined in WT and IL-7−/− pre-proB cells. Open histograms represent the expression of IL-7Rα or _γ_c. The shaded histograms represent the negative control stained with isotype-matched irrelevant antibodies. The number in the plot indicates the mean fluorescence intensity (MFI) value. The mean ± S.D. from 4 mice was calculated and indicated in the FACS plots. (B) Expression of essential components of IL-7R signaling was examined by semi-quantitative RT-PCR. WT or IL-7−/− pre-proB cells (1.5×104) were used for RNA purification. Synthesized cDNA was serially diluted by 5-fold and used for PCR amplification of each gene indicated. (C) CIS is normally upregulated in both WT and IL-7−/− pre-proB cells after IL-7 stimulation. Pre-proB cells (2.0×104) were purified from either WT or IL-7−/− mice and pre-cultured in complete medium in the absence of IL-7 for 12 hrs. After this cytokine starvation, cells were further incubated with or without 50 ng/ml IL-7 for 2 hrs. After RNA purification and cDNA synthesis, CIS expression was examined by semi-quantitative PCR. (D) CD11c and CD19 expression after 6 day culture of WT or IL-7−/− pre-proB cells. (E) Expression of various DC markers on the CD11c+ cells derived from IL-7−/− pre-proB cells. Open histograms represent the expression level of various markers and the shaded histograms represent the negative control stained with isotype-matched irrelevant Abs. Representative data from two independent experiments were shown.
Figure 5. B cell potential of IL-7−/− CLPs
in vitro. (A) The frequency of cells that give rise to CD19+ proB cells in the CLP population derived from WT and IL- 7−/− mice. Various numbers of CLPs from WT and IL-7−/− mice were plated in wells of 96 well plates and cultured as described in Figure 2A. Wells containing B220+CD19+ cells were counted as a positive well. The limiting numbers in this assay are 1 in 19 for WT pre-proB cells and 1 in 132 for IL-7−/− pre-proB cells. (B) The kinetic analysis of B cell development from WT or IL-7−/− CLPs. CLPs (2×103 cells/well) from either WT or IL-7−/− mice were cultured as described in Figure 3A. In the plots, B220−CD19−, B220+CD19−, and B220+CD19+ cells are CLP, pre-proB, and proB cells, respectively. The period of time in the culture is indicated at the top of the FACS plots. Reanalysis of freshly isolated CLPs before culture was shown as day 0. The mean percentage ± S.D. from more than 6 samples from 2 independent experiments was indicated in the FACS plots.
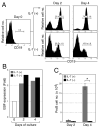
Figure 6. EBF expression level in WT and IL-7−/− pre-proB cells
(A) EBF expression in the CLP (grey bar) and pre-proB (black bar) populations derived from WT or IL-7−/− mice was examined by quantitative PCR. EBF expression in whole BM was arbitrary defined as unit one and the mean value of three independent samples is shown. The same results were obtained by two independent experiments. (B) EBF expression in WT and IL-7−/− pre-proB cells (2.0×104) was examined before and after IL-7 stimulation by semi-quantitative PCR. After RNA purification and first strand synthesis, cDNAs were serially diluted by 5-fold and subjected to PCR. ProB cell markers such as CD19 and BP-1 were not upregulated during the culture (17). (C) Pax5 expression in IL-7−/−pre-proB cells after IL-7 stimulation. IL-7−/− pre-proB cells were stimulated with IL-7 as described above for the period indicated in the figure. Pax5 expression in each sample was measured by RT-PCR. β-actin expression was also examined as a loading control. WT pre-proB cells were used as a positive control for Pax5.
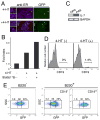
Figure 7. EBF plays an indispensable role in the transition from the pre-proB to proB stage
(A) 293T cells were infected with recombinant viruses with MSCV-EBF-ER-IRES-GFP and cultured in the absence (top) and presence (bottom) of 4-HT (1 μM). Only GFP+ (green) cells were brightly stained with anti-ER (red) as indicated by arrowheads. Therefore, only EBF-ER was detected by anti-ER staining in this figure. EBF-ER was located in the cytoplasm and nucleus in the absence and presence of 4-HT as shown in the left panels. Nuclei were stained with DAPI (blue). (B) EBF activity was measured by the reporter assay with pPax5-luc in 293T cells. EBF can positively regulate the promoter activity of the Pax5 gene (37). This EBF function was observed only when 4-HT was added into the culture. Synergistic effects of EBF-ER with the constitutively active form of Stat5 (Stat5(1*6)) (45) was also observed in the presence of 4-HT. (C) IL-7 expression in OP9 and PA6 cells was examined by RT-PCR. (D) Maturation of pre-proB cells to proB cells in the presence of ectopic EBF and absence of IL-7Rα. After introduction of EBF-ER into MPPs derived from IL-7Rα−/− mice, EBF-ER+ pre-proB cells were purified from EBF-ER+ MPPs that had been cultured for 4 days. EBF-ER+ pre-proB cells were further cultured for 2–3 days in the absence (left) and presence (right) of 4-HT (0.3 μM). A representative result from at least 5 experiments is shown. (E) EBF expression levels required for the transition from the pre-proB to proB stage. VCAM-1+ MPPs (IL-7Rα−) from WT mice were infected with recombinant retrovirus derived from MSCV-EBF-IRES-GFP vectors (17). After infection, GFP+ cells were purified in order to reduce the number of non-infected cells. These GFP+ cells were cultured for 3 days on PA6 stromal cells in the presence of SCF and Flt3L. PA6 stromal cells do not produce any IL-7 (36). GFP expression levels in B220+CD19− pre-proB cells (center panel) and B220+CD19+ proB cells (right panel) as well as B220−CD19−cells (left panel), most of which were Mac-1+ myeloid cells, were examined by FACS.
Figure 8. Dispensability of IL-7 in the transition from the pre-proB to proB stage
(A) Requirement of IL-7 in the stage transition from the pre-proB to proB stage. Pre-proB cells were purified from WT mice and cultured on PA6 in the presence or absence of IL-7. Although CD19 expression was observed after the culture in the absence of IL-7, CD19 expression levels were constantly lower than the cells cultured with IL-7. The mean ± S.D. from more than 6 samples from two independent analyses were indicated in the FACS plots. (B) EBF expression level was examined in proB cells derived from pre-proB cells after the culture shown in Figure 6C. B220+CD19+ proB cells were sorted from the culture of WT pre-proB cells in the presence (grey) and absence (black) of IL-7. EBF expression was examined by quantitative RT-PCR. The fold expression of EBF in proB cells was calculated against the EBF expression in WT pre-proB cells (day 0). (C) ProB cell numbers after the culture in the presence (grey) and absence (black) of IL-7. The bars are shown as means of triplicate wells ± S.D. *, p < 0.001, calculated by student’s t test.
Similar articles
- IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF.
Kikuchi K, Lai AY, Hsu CL, Kondo M. Kikuchi K, et al. J Exp Med. 2005 Apr 18;201(8):1197-203. doi: 10.1084/jem.20050158. J Exp Med. 2005. PMID: 15837809 Free PMC article. - IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors.
Tsapogas P, Zandi S, Åhsberg J, Zetterblad J, Welinder E, Jönsson JI, Månsson R, Qian H, Sigvardsson M. Tsapogas P, et al. Blood. 2011 Aug 4;118(5):1283-90. doi: 10.1182/blood-2011-01-332189. Epub 2011 Jun 7. Blood. 2011. PMID: 21652681 - Suppression of B lymphopoiesis at a lymphoid progenitor stage in adult rabbits.
Kalis SL, Zhai SK, Yam PC, Witte PL, Knight KL. Kalis SL, et al. Int Immunol. 2007 Jun;19(6):801-11. doi: 10.1093/intimm/dxm048. Epub 2007 May 13. Int Immunol. 2007. PMID: 17502309 - Transcriptional regulation of early B cell development.
Northrup DL, Allman D. Northrup DL, et al. Immunol Res. 2008;42(1-3):106-17. doi: 10.1007/s12026-008-8043-z. Immunol Res. 2008. PMID: 18818886 Review. - The key role of IL-7 in lymphopoiesis.
Ceredig R, Rolink AG. Ceredig R, et al. Semin Immunol. 2012 Jun;24(3):159-64. doi: 10.1016/j.smim.2012.02.004. Epub 2012 Mar 14. Semin Immunol. 2012. PMID: 22421573 Review.
Cited by
- HCV infection activates the proteasome via PA28γ acetylation and heptamerization to facilitate the degradation of RNF2, a catalytic component of polycomb repressive complex 1.
Kasai H, Yamashita A, Akaike Y, Tanaka T, Matsuura Y, Moriishi K. Kasai H, et al. mBio. 2024 Nov 13;15(11):e0169124. doi: 10.1128/mbio.01691-24. Epub 2024 Sep 27. mBio. 2024. PMID: 39329491 Free PMC article. - Hematopoietic stem cell-derived Tregs are essential for maintaining favorable B cell lymphopoiesis following posttransplant cyclophosphamide.
Sumii Y, Kondo T, Ikegawa S, Fukumi T, Iwamoto M, Nishimura MF, Sugiura H, Sando Y, Nakamura M, Meguri Y, Matsushita T, Tanimine N, Kimura M, Asada N, Ennishi D, Maeda Y, Matsuoka KI. Sumii Y, et al. JCI Insight. 2023 Apr 24;8(8):e162180. doi: 10.1172/jci.insight.162180. JCI Insight. 2023. PMID: 37092551 Free PMC article. - Hematopoietic stem cells preferentially traffic misfolded proteins to aggresomes and depend on aggrephagy to maintain protein homeostasis.
Chua BA, Lennan CJ, Sunshine MJ, Dreifke D, Chawla A, Bennett EJ, Signer RAJ. Chua BA, et al. Cell Stem Cell. 2023 Apr 6;30(4):460-472.e6. doi: 10.1016/j.stem.2023.02.010. Epub 2023 Mar 21. Cell Stem Cell. 2023. PMID: 36948186 Free PMC article. - IL7Rα, but not Flk2, is required for hematopoietic stem cell reconstitution of tissue-resident lymphoid cells.
Worthington AK, Cool T, Poscablo DM, Hussaini A, Beaudin AE, Forsberg EC. Worthington AK, et al. Development. 2022 Apr 15;149(8):dev200139. doi: 10.1242/dev.200139. Epub 2022 Jan 24. Development. 2022. PMID: 35072209 Free PMC article. - The Broad Immunomodulatory Effects of IL-7 and Its Application In Vaccines.
Huang J, Long Z, Jia R, Wang M, Zhu D, Liu M, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Tian B, Mao S, Ou X, Sun D, Gao Q, Cheng A. Huang J, et al. Front Immunol. 2021 Dec 10;12:680442. doi: 10.3389/fimmu.2021.680442. eCollection 2021. Front Immunol. 2021. PMID: 34956167 Free PMC article. Review.
References
- Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, Yamamura K, Sugamura K. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. - PubMed
- Leonard WJ, Shores EW, Love PE. Role of the common cytokine receptor gamma chain in cytokine signaling and lymphoid development. Immunol Rev. 1995;148:97–114. - PubMed
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA098129-03/CA/NCI NIH HHS/United States
- R01 AI056123/AI/NIAID NIH HHS/United States
- R01 CA098129-01A2/CA/NCI NIH HHS/United States
- T32 AI052077/AI/NIAID NIH HHS/United States
- CA098129/CA/NCI NIH HHS/United States
- R01 CA098129/CA/NCI NIH HHS/United States
- R01 CA098129-02/CA/NCI NIH HHS/United States
- R01 AI056123-01A1/AI/NIAID NIH HHS/United States
- R01 AI056123-05/AI/NIAID NIH HHS/United States
- R01 CA098129-05/CA/NCI NIH HHS/United States
- AI52077/AI/NIAID NIH HHS/United States
- AI056123/AI/NIAID NIH HHS/United States
- R01 AI056123-04/AI/NIAID NIH HHS/United States
- R01 AI056123-02/AI/NIAID NIH HHS/United States
- R01 AI056123-03/AI/NIAID NIH HHS/United States
- R01 CA098129-04/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases