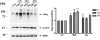Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells - PubMed (original) (raw)
Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells
Tereza Vogiatzi et al. J Biol Chem. 2008.
Abstract
Alpha-synuclein (ASYN) is crucial in Parkinson disease (PD) pathogenesis. Increased levels of wild type (WT) ASYN expression are sufficient to cause PD in humans. The manner of post-transcriptional regulation of ASYN levels is controversial. Previously, we had shown that WT ASYN can be degraded by chaperone-mediated autophagy (CMA) in isolated liver lysosomes. Whether this occurs in a cellular and, in particular, in a neuronal cell context is unclear. Using a mutant ASYN form that lacks the CMA recognition motif and RNA interference against the rate-limiting step in the CMA pathway, Lamp2a, we show here that CMA is indeed involved in WT ASYN degradation in PC12 and SH-SY5Y cells, and in primary cortical and midbrain neurons. However, the extent of involvement varies between cell types, potentially because of differences in compensatory mechanisms. CMA inhibition leads to an accumulation of soluble high molecular weight and detergent-insoluble species of ASYN, suggesting that CMA dysfunction may play a role in the generation of such aberrant species in PD. ASYN and Lamp2a are developmentally regulated in parallel in cortical neuron cultures and in vivo in the central nervous system, and they physically interact as indicated by co-immunoprecipitation. In contrast to previous reports, inhibition of macroautophagy, but not the proteasome, also leads to WT ASYN accumulation, suggesting that this lysosomal pathway is also involved in normal ASYN turnover. These results indicate that CMA and macroautophagy are important pathways for WT ASYN degradation in neurons and underline the importance of CMA as degradation machinery in the nervous system.
Figures
FIGURE 1.
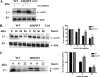
ΔDQ/WT ASYN exhibits slower turnover compared with WT ASYN in PC12 cells. A, generation of stable inducible Tet-Off PC12 cell lines overexpressing WT ASYN and ΔDQ/WT. The cells were cultured in the presence (+) or absence (–) of dox (2 μg/ml) for 4 days and assayed for ASYN expression with the C20 polyclonal Ab. ERK Ab is used as a loading control. B and C, overexpressed ΔDQ/WT ASYN displays a slower rate of degradation compared with WT ASYN. B, ΔDQ/WT and WT PC12 cell lines were cultured in the absence of dox for 5 days, and then dox (2 μg/ml) was added at the indicated times. _Left,representative immunoblot of ASYN levels. Right, quantification of rate of overexpressed ΔDQ/WT and WT ASYN turnover after dox addition.Ctrl, control. C, ΔDQ/WT and WT ASYN cell lines were cultured in the absence of dox for 5 days. The cells were then labeled with [35S]cysteine/methionine and pulse-chased for 0, 8, and 22 h. ASYN was immunoprecipitated from hot lysates with C20 Ab, and its levels were assessed by autoradiography. Left, a representative pulse-chase experiment is shown. The band corresponding to ASYN is depicted by the_arrow. The asterisk indicates an irrelevant band.Right, quantification of the turnover of ΔDQ/WT and WT ASYN. All data are presented as the relative OD values of each time point relative to time point 0. The graphs represent the mean ± S.E. of three independent experiments. (*, p < 0.05, Student's t test comparing ΔDQ/WT to WT ASYN).
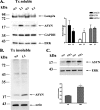
FIGURE 2.
Down-regulation of Lamp2a in PC12 cells leads to slower turnover of WT ASYN. A, transient transfection of rat Lamp2a siRNA (L1), effectively down-regulates endogenous Lamp2a but has no effect on the steady state levels of overexpressed WT ASYN. PC12 cells expressing WT ASYN were cultured in the absence of dox for 5 days and then were transiently transfected with L1 or scr siRNA. 48 h later, cells were lysed and assayed for ASYN and Lamp2a. Left, representative immunoblot of Lamp2a and ASYN.Middle and right, quantification of Lamp2a (middle) and ASYN (right) levels in cells transfected with L1 compared with cells transfected with scr siRNA. Results are expressed as the ratio to OD values of the corresponding controls, and data are presented as mean of + S.E. of 9 (for Lamp2a) and 6 (ASYN) independent experiments. B–D, WT ASYN displays a slower turnover rate in cells transfected with L1 compared with cells transfected with scr siRNA. B, WT ASYN cells were cultured without dox for 5 days and next transfected with L1 or scr siRNA, and 48 h later dox was added. At successive time points after dox addition, cells were examined for ASYN levels by immunoblotting. Left, representative immunoblot of ASYN. Right, quantification of turnover rate of WT ASYN after dox addition. C, WT ASYN cells were treated as in B_and examined for ASYN levels by immunoblotting. Left panel, the detergent (Triton X-100 (Tx))-soluble L1 or scr siRNA-treated samples were run on 4–12% BisTris NuPAGE gels and assayed by Western blot for ASYN and ERK (loading control). High molecular weight ASYN species in the Lamp2a down-regulated (L1)-treated samples are indicated by a_brace. The asterisk indicates an irrelevant band. Right panel, the detergent-insoluble pellets from the L1 or scr siRNA-treated samples were solubilized in SDS sample buffer, run on 12% gel, and assayed by Western blot for ASYN and β-actin (loading control). Representative Western blots from three separate experiments are shown. D, WT ASYN cell lines were cultured in the absence of dox for 5 days. Cultures were treated with siRNAs as in B and labeled with [35S]cysteine/methionine for pulse-chase. ASYN was immunoprecipitated from hot lysates with C20 ASYN Ab, and its levels were assessed by autoradiography (indicated by the arrow). _Left,representative pulse-chase is shown. Right, quantification of the turnover of WT ASYN in cells transfected with L1 or scr siRNA. All data are presented as the relative OD values of each time point relative to time point 0. The graphs represent the mean ± S.E. of three independent experiments. (*, p < 0.05; ***, p < 0.001, Student's_t test comparing L1 with the control scr).
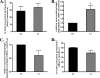
FIGURE 3.
Inhibition of macroautophagy increases endogenous (A) and human overexpressed (B) WT ASYN levels in PC12 cells. Cells were cultured in the presence of 3-MA (10 m
m
) or epoxomicin (1 μ
m
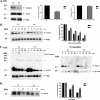
) for 14 h. Untreated cells were used as a control (Ctrl). Cell lysates were assessed by Western immunoblotting for ASYN levels. Left, representative immunoblot of ASYN. _Right,_quantification of endogenous (A) or overexpressed (B) ASYN levels after 3-MA or epx addition, compared with control. Results are expressed as the ratio of OD values to the corresponding controls, and data are presented as mean ± S.E. of six (for 3-MA) or three (B, for epx) independent experiments. (*, p < 0.05; ***, p< 0.001, Student's t test comparing 3-MA or epx with the control).
FIGURE 4.
Involvement of CMA in degradation of ASYN in SH-SY5Y cell lines. A, generation of stable inducible Tet-Off SH-SY5Y cell lines overexpressing ΔDQ/WT and WT ASYN. Cells were treated with or without 3 μg/ml dox for 4 days, and lysates were used for ASYN immunoblotting.B and C, overexpressed ΔDQ ASYN displays a slower rate of degradation compared with WT ASYN. B, ΔDQ/WT and WT ASYN SH-SY5Y cell lines were cultured in the absence of dox for 5 days, and then dox was added at the indicated times. Left, representative immunoblot of ASYN levels. Right, quantification of rate of turnover of overexpressed ΔDQ/WT and WT ASYN after dox addition. _C,_ΔDQ/WT and WT ASYN SH-SY5Y cell lines were cultured in the absence of dox for 5 days. The cells were then labeled with [35S]cysteine/methionine for pulse-chase. ASYN was immunoprecipitated with C20 ASYN Ab, and its levels were assessed by autoradiography. ERK immunoprecipitation was used as negative control (Ctrl). Left, representative pulse-chase is shown.Right, quantification of the turnover of ΔDQ/WT and WT ASYN. All results are expressed as the ratio of OD values to the corresponding controls, and all data are presented as a percent of each time point compared with the value at time point 0. (*, p < 0.05; **, p < 0.01; ***, p < 0.001, Student's t test comparing ΔDQ/WT to WT ASYN).
FIGURE 5.
Expression of ASYN and Lamp2a in rat cortical cultures. _A,_levels of ASYN and of Lamp2a increase in parallel during _in vitro_maturation of rat cortical neurons. Immunoblot probed with Syn 1 ASYN Ab, Lamp2a, and ERK (loading control) Abs from cortical cultures collected from day 0 to day 12 after plating. B, effect of different inhibitors on the degradation of long lived proteins on rat cortical cultures. [3H]Leucine-labeled cortical cultures (day 7) supplemented with or without NH4Cl (20 m
m
) or 3-MA (10 m
m
) were assayed for long lived protein degradation as described under “Experimental Procedures.” Values are the mean ± S.E. of three independent experiments, and within each experiment triplicate samples per condition were assessed (*, p < 0.05, for Student's _t_test, comparing 3-MA or NH4Cl with the control). C, ASYN immunoprecipitated from rat cortical cultures. Cultures (day 7) were labeled for 24 h with a [35S]methionine/cysteine and pulse-chased for 0 and 14 h. ASYN was immunoprecipitated from hot lysates with Syn 1 ASYN antibody. A representative pulsechase experiment is shown. D, ASYN interacts with Lamp2a receptor in cortical cultures. Lysates from cortical cultures (day 7) were immunoprecipitated (IP) with Syn 1 ASYN antibody and GAPDH. ERK immunoprecipitation was used as a negative control for nonspecific IgG-associated bands. The samples were immunoblotted for Lamp2a, ASYN, and GAPDH antibodies. ASYN immunoprecipitated Lamp2a from cortical cultures co-migrates with the Lamp2a-specific band in the same cortical lysate (bottom panel). Representative Western blots from three independent experiments are presented.
FIGURE 6.
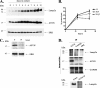
Lentiviral down-regulation of Lamp2a significantly increases endogenous ASYN protein levels in rat cortical neurons. CMA impairment via Lamp2a silencing results in endogenous ASYN accumulation. Primary rat cortical neurons were transduced with lentiviruses (m.o.i. 5) expressing Lamp2a (L1) or scr siRNA for 24 h. After 48 and 72 h, cells were assayed by Western blot for Lamp2a, ASYN, GFP (infection control), and ERK (loading control) levels.A, representative image of rat cortical neurons 72 h post-infection with the L1 lentivirus that contains a CMV-EGFP reporter cassette to monitor infection is shown. B, left, representative Western blots from 12 separate experiments are shown. Right, densitometric analysis of the levels of Lamp2a and ASYN. C, detergent (Triton X-100 (Tx)-insoluble pellets from the L1 or scr siRNA-treated samples were solubilized in SDS sample buffer and assayed by Western blot. Representative Western blots from three separate experiments are shown. D, ASYN immunostaining in rat cortical neurons 72 h post-infection with the scr or L1 lentiviruses. ASYN immunostaining in nontransduced (nt) neurons is also presented. The same microscopy settings were used in all cases. L1 infected neurons display increased ASYN immunofluorescence. No frank inclusions were observed. E, mRNA levels of ASYN are not altered following down-regulation of Lamp2a. 48 and 72 h post-infection RNA was extracted, and RT-PCR for ASYN and β-actin was performed. Representative images from three separate experiments are shown in the left panel. Densitometric analysis of the RT-PCR products, expressed as ASYN: β-actin ratio (arbitrary units), is presented in the right panel. All results are presented as the ratio to OD values of the corresponding controls, and data are presented as mean ± S.E. (**, p < 0.01; ***,p < 0.001, one way ANOVA followed by the Student-Newman-Keuls' test, comparing L1 to scr).
FIGURE 7.
Macroautophagy participates in the degradation of endogenous ASYN in rat cortical neurons. Cultures were incubated with 3-MA (10 m
m
) or epx (50 n
m
). At the end of the incubation the cells were assayed for ASYN, ubiquitin (ubiq), and ERK (loading control) levels by Western blot. Ctrl, control. Representative Western blots from nine independent experiments are shown in the left panels, and densitometric analysis of the levels of ASYN is shown in the right panel. Results are expressed as the ratio to OD values of the corresponding controls, and data are presented as mean ± S.E. of nine independent experiments. (**, p < 0.01; ***, p < 0.001, one way ANOVA followed by the Student-Newman-Keuls' test, comparing 3-MA or epoxomicin with the control).
FIGURE 8.
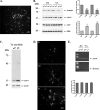
Blockade of CMA or macroautophagy increases endogenous ASYN protein levels in postnatal ventral midbrain neurons. Three days after plating, postnatal ventral midbrain neurons (P1) were transduced with lentiviruses (m.o.i. 5) expressing Lamp2a (L1) or scr siRNA for 24 h. After 72 h, cells were assayed by Western blot for Lamp2a, ASYN, GAPDH, and ERK (loading control) levels. A, left, representative Western blots from three separate experiments are shown. Right, densitometric analysis of the levels of Lamp2a, ASYN, and GAPDH. B, detergent (Triton X-100)-insoluble pellets from the L1 or scr-treated samples were solubilized in SDS sample buffer and assayed by Western blot for ASYN and β-actin (loading control) levels. Representative Western blots from three separate experiments are shown. C, cultures were incubated with 3-MA (10 m
m
) for 24 h. Untreated cells were used as a control (ctrl). Cell lysates were assessed by Western immunoblotting for ASYN levels. Upper panel, representative immunoblot of ASYN. Bottom panel, quantification of endogenous ASYN levels after 3-MA addition, compared with control. All results are expressed as the ratio to OD values of the corresponding controls, and data are presented as mean ± S.E. of three independent experiments. (*, p < 0.05; **, p < 0.01, one way ANOVA followed by the Student-Newman-Keuls' test).
FIGURE 9.
Effects of CMA down-regulation on macroautophagy. PC12 cells transiently transfected with L1 or scr siRNA were labeled with [3H]leucine for 48 h (2 μCi/ml). Cells were treated with or without NH4Cl or 3-MA and degraded proteins were assayed 14 h later. A, rate of total long lived protein degradation in PC12 cells transfected with L1 or scrambled siRNA. B, rate of macroautophagic degradation (inhibitable by 3-MA) in PC12 cells transfected with L1 or scrambled siRNA. C, rate of non-macroautophagic lysosomal degradation (defined as the difference between NH4Cl-sensitive and 3-MA-sensitive long lived protein degradation) in PC12 cells transfected with L1 or scrambled siRNA. All presented data are the mean of four independent experiments, and within each experiment triplicate samples per condition were assessed. D, Lamp2a down-regulation results in decreased long lived protein degradation. Cortical cultures were transduced with lentiviruses expressing L1 or scr siRNA for 24 h. and then labeled with [3H]leucine for 24 h. Protein degradation was assayed 12 h later. All presented data are the mean of three independent experiments, and within each experiment triplicate samples per condition were assessed. (**,p < 0.01, *, p < 0.05, for Student's t test comparing between cells transduced with the L1 or scr siRNA lentiviruses.)
FIGURE 10.
Expression and interaction of ASYN and Lamp2a in rats and WT and A53T transgenic mice. A, Lamp2a and ASYN display a similar expression pattern during maturation in rat cortex. Proteins from embryonic day 16 to postnatal day 21 were lysed and immunoblotted for Lamp2a, ASYN, and ERK (loading control). B, Lamp2a and ASYN are expressed in the cortex and midbrain of WT and A53T transgenic mice. C, Lamp2a co-immunoprecipitates with rat (WT) and mouse (WT and A53T) ASYN. Lysates from rat cortex and midbrains of WT and A53T mice were immunoprecipitated (IP) with Syn 1 ASYN and ERK antibodies (negative control) and then immunoblotted with Lamp2a and Syn 1 ASYN antibodies. ASYN immunoprecipitated Lamp2a from rat brain co-migrates with the Lamp2a-specific band in the same brain extract (right panel). Representative Western blots from three independent experiments are presented.
Similar articles
- Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy.
Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Xilouri M, et al. PLoS One. 2009;4(5):e5515. doi: 10.1371/journal.pone.0005515. Epub 2009 May 13. PLoS One. 2009. PMID: 19436756 Free PMC article. - Age-dependent accumulation of oligomeric SNCA/α-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA).
Ho PW, Leung CT, Liu H, Pang SY, Lam CS, Xian J, Li L, Kung MH, Ramsden DB, Ho SL. Ho PW, et al. Autophagy. 2020 Feb;16(2):347-370. doi: 10.1080/15548627.2019.1603545. Epub 2019 Apr 14. Autophagy. 2020. PMID: 30983487 Free PMC article. - alpha-synuclein degradation by autophagic pathways: a potential key to Parkinson's disease pathogenesis.
Xilouri M, Vogiatzi T, Vekrellis K, Stefanis L. Xilouri M, et al. Autophagy. 2008 Oct;4(7):917-9. doi: 10.4161/auto.6685. Epub 2008 Oct 26. Autophagy. 2008. PMID: 18708765 - A new perspective in Parkinson's disease, chaperone-mediated autophagy.
Li B, Zhang Y, Yuan Y, Chen N. Li B, et al. Parkinsonism Relat Disord. 2011 May;17(4):231-5. doi: 10.1016/j.parkreldis.2010.12.008. Epub 2011 Jan 7. Parkinsonism Relat Disord. 2011. PMID: 21215675 Review. - Autophagy and Alpha-Synuclein: Relevance to Parkinson's Disease and Related Synucleopathies.
Xilouri M, Brekk OR, Stefanis L. Xilouri M, et al. Mov Disord. 2016 Feb;31(2):178-92. doi: 10.1002/mds.26477. Epub 2016 Jan 27. Mov Disord. 2016. PMID: 26813776 Review.
Cited by
- Lysosomal lipid alterations caused by glucocerebrosidase deficiency promote lysosomal dysfunction, chaperone-mediated-autophagy deficiency, and alpha-synuclein pathology.
Navarro-Romero A, Fernandez-Gonzalez I, Riera J, Montpeyo M, Albert-Bayo M, Lopez-Royo T, Castillo-Sanchez P, Carnicer-Caceres C, Arranz-Amo JA, Castillo-Ribelles L, Pradas E, Casas J, Vila M, Martinez-Vicente M. Navarro-Romero A, et al. NPJ Parkinsons Dis. 2022 Oct 6;8(1):126. doi: 10.1038/s41531-022-00397-6. NPJ Parkinsons Dis. 2022. PMID: 36202848 Free PMC article. - An Update on Autophagy in Prion Diseases.
López-Pérez Ó, Badiola JJ, Bolea R, Ferrer I, Llorens F, Martín-Burriel I. López-Pérez Ó, et al. Front Bioeng Biotechnol. 2020 Aug 27;8:975. doi: 10.3389/fbioe.2020.00975. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32984276 Free PMC article. Review. - Proteostasis Disturbances and Inflammation in Neurodegenerative Diseases.
Sonninen TM, Goldsteins G, Laham-Karam N, Koistinaho J, Lehtonen Š. Sonninen TM, et al. Cells. 2020 Sep 28;9(10):2183. doi: 10.3390/cells9102183. Cells. 2020. PMID: 32998318 Free PMC article. Review. - Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism.
Suntharalingam A, Abisambra JF, O'Leary JC 3rd, Koren J 3rd, Zhang B, Joe MK, Blair LJ, Hill SE, Jinwal UK, Cockman M, Duerfeldt AS, Tomarev S, Blagg BS, Lieberman RL, Dickey CA. Suntharalingam A, et al. J Biol Chem. 2012 Nov 23;287(48):40661-9. doi: 10.1074/jbc.M112.384800. Epub 2012 Oct 3. J Biol Chem. 2012. PMID: 23035116 Free PMC article. - α-Synuclein binds the K(ATP) channel at insulin-secretory granules and inhibits insulin secretion.
Geng X, Lou H, Wang J, Li L, Swanson AL, Sun M, Beers-Stolz D, Watkins S, Perez RG, Drain P. Geng X, et al. Am J Physiol Endocrinol Metab. 2011 Feb;300(2):E276-86. doi: 10.1152/ajpendo.00262.2010. Epub 2010 Sep 21. Am J Physiol Endocrinol Metab. 2011. PMID: 20858756 Free PMC article.
References
- Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., and Riess, O. (1998) Nat. Genet. 18106 –108 - PubMed
- Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997) Science 2762045 –2047 - PubMed
- Singleton, A., and Gwinn-Hardy, K. (2004) Lancet 3641105 –1107 - PubMed
- Maraganore, D. M., de Andrade, M., Elbaz, A., Farrer, M. J., Ioannidis, J. P., Kruger, R., Rocca, W. A., Schneider, N. K., Lesnick, T. G., Lincoln, S. J., Hulihan, M. M., Aasly, J. O., Ashizawa, T., Chartier-Harlin, M. C., Checkoway, H., Ferrarese, C., Hadjigeorgiou, G., Hattori, N., Kawakami, H., Lambert, J. C., Lynch, T., Mellick, G. D., Papapetropoulos, S., Parsian, A., Quattrone, A., Riess, O., Tan, E. K., and Van Broeckhoven, C. (2006) J. Am. Med. Assoc. 296661 –670 - PubMed
- Bennett, M. C., Bishop, J. F., Leng, Y., Chock, P. B., Chase, T. N., and Mouradian, M. M. (1999) J. Biol. Chem. 27433855 –33858 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous