The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C - PubMed (original) (raw)
. 2008 Jul 23;27(14):1932-43.
doi: 10.1038/emboj.2008.120. Epub 2008 Jun 19.
Weiming Ouyang, Hua Wei, Nelyn Soto, Adam Lazorchak, Christine Gould, Carolyn Lowry, Alexandra C Newton, Yuxin Mao, Robert Q Miao, William C Sessa, Jun Qin, Pumin Zhang, Bing Su, Estela Jacinto
Affiliations
- PMID: 18566586
- PMCID: PMC2486276
- DOI: 10.1038/emboj.2008.120
The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C
Valeria Facchinetti et al. EMBO J. 2008.
Abstract
The target of rapamycin (TOR), as part of the rapamycin-sensitive TOR complex 1 (TORC1), regulates various aspects of protein synthesis. Whether TOR functions in this process as part of TORC2 remains to be elucidated. Here, we demonstrate that mTOR, SIN1 and rictor, components of mammalian (m)TORC2, are required for phosphorylation of Akt and conventional protein kinase C (PKC) at the turn motif (TM) site. This TORC2 function is growth factor independent and conserved from yeast to mammals. TM site phosphorylation facilitates carboxyl-terminal folding and stabilizes newly synthesized Akt and PKC by interacting with conserved basic residues in the kinase domain. Without TM site phosphorylation, Akt becomes protected by the molecular chaperone Hsp90 from ubiquitination-mediated proteasome degradation. Finally, we demonstrate that mTORC2 independently controls the Akt TM and HM sites in vivo and can directly phosphorylate both sites in vitro. Our studies uncover a novel function of the TOR pathway in regulating protein folding and stability, processes that are most likely linked to the functions of TOR in protein synthesis.
Figures
Figure 1
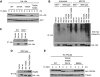
Identification of the TM phosphorylation in Akt and PKCα/β as a novel SIN1-regulated, growth factor-independent event. (A) Akt from either starved or growth factor-stimulated SIN1-deficient cells migrates faster than its counterpart in wild-type cells on an SDS–PAGE gel. Wild-type and SIN1−/− MEFs were grown in complete medium (medium), or starved of serum overnight and amino acids for 1 h (starved), or starved then restimulated with insulin for 30 min as indicated. Total cellular extracts were prepared for immunoblot analysis for total Akt and phosphorylated Akt at Thr308 in the activation loop (p-Akt A-loop) and at Ser473 in the HM (p-Akt HM). (B) The faster migrating Akt band in SIN1-deficient cells is due to a defective phosphorylation unrelated to the A-loop or HM site phosphorylation. Wild-type and SIN1−/− MEFs were starved as described in 1A. Total cellular extracts were prepared and treated with or without lambda protein phosphatase (λ-PPase) for 30 min at 30 °C before analysis of total Akt and phospho-Akt as described in panel A. (C) Sequence alignment of the TM region of various members of AGC kinases in yeast, Drosophila and mammals. Underlined residue indicates the phosphorylation site in the TM. Numbering is based on human sequences except as indicated; D.m., Drosophila melanogaster; S.c., Saccharomyces cerevisiae. (D) TM phosphorylation of Akt and PKCα/βII is defective in SIN1−/− cells. SIN1−/− MEFs were infected with a control (SIN1−/− vector) or HA–SIN1 (SIN1−/− HA–SIN1) retroviral expression vectors as indicated. Total cell extracts were prepared from starved or starved then insulin stimulated wild-type MEFs or retroviral-infected cells as indicated and analysed by immunoblotting for total Akt, PKCα and phospho-Akt and phospho-PKCα/βII. ERK2 and PKCθ levels were used as loading control.
Figure 2
The TORC2 components are essential for Akt and PKCα/βII TM phosphorylation. (A) The TM phosphorylation of Akt and conventional PKC is insensitive to short-term treatment with rapamycin and wortmannin. WT and SIN1−/− MEFs were starved as described in Figure 1A, then treated with vehicle (−), rapamycin (50 nM) or wortmannin (100 nM) for 30 min, followed by insulin stimulation for another 30 min as indicated. Phosphorylation and total protein levels were determined by immunoblotting as described in Figure 1B. (B) The mTORC2 component rictor is essential for the Akt and PKCα/βII TM site phosphorylation. WT and rictor−/− MEFs were starved, stimulated and analysed for phosphorylation and total protein levels by immunoblotting as described in panel A. ERK2 was assayed as a loading control. (C) Knockdown of mTOR expression attenuates the Akt and PKCα/βII TM phosphorylation. NIH3T3 cells were transfected with varying amounts (0.2, 0.6 and 1.0 μg) of either vector control or _mTOR_-siRNA-expressing plasmid DNA as indicated. Total cell extracts were prepared and phosphorylation was analysed by immunoblotting. All lanes from each antibody-blotted panel come from the same blot. (D) Disruption of mTORC2 by prolonged rapamycin treatment decreases TM phosphorylation. Wild-type MEFs were incubated with rapamycin (Rap; 100 nM) for the indicated number of hours (h). SIN1 was immunoprecipitated and associated mTOR and rictor were detected by immunoblotting. Total extracts were analysed for phosphorylation and for total protein levels by immunoblotting.
Figure 3
The TM site phosphorylation is required for Akt and PKCα/βII stability and PKCα maturation. (A) Protein levels of Akt and PKCα are diminished in different SIN1−/− MEF lines. Individual WT and SIN1−/− MEFs established from six different embryos (1–6) were analysed by immunoblotting for Akt, PKCα and PKCθ levels. ERK2 and tubulin expression was assayed as a loading control. (B, C) Akt is degraded through the ubiquitination pathway in SIN1−/− cells. WT and SIN1−/− MEFs were transfected with myc-ubiquitin or co-transfected with myc-ubiquitin and HA–Akt-expressing plasmid DNA. After 36 h, transfected cells were either untreated or treated with MG132 (10 μM) for another 12 h before harvest. Exogenous HA–Akt (B) or endogenous Akt (C) were immunoprecipitated and further analysed for ubiquitination by immunoblotting with an anti-myc antibody. Immunoprecipitated Akt or HA–Akt levels were also determined. (D) Defective PKCα maturation in SIN1−/− MEFS. WT and SIN1−/− MEFs were metabolically labelled with [35S] methionine/cysteine for 7 min and chased at different time points (min) as indicated before harvesting. Endogenous PKCα was immunoprecipitated from detergent-solubilized lysates with an anti-PKCα antibody, separated by SDS–PAGE, and visualized by autoradiography (top panel). Newly synthesized, unphosphorylated PKCα is indicated by the dash (−); phosphorylated and mature PKCα is indicated by a double asterisk (**). The bottom panel shows an immunoblot analysis of total PKCα.
Figure 4
The remaining Akt and PKCα in SIN1−/− cells are protected from degradation by the molecular chaperone Hsp90. (A, B) Inhibition of Hsp90 with 17-AAG destabilizes Akt and PKCα dramatically in SIN1−/− or rictor−/− cells but not in wild-type cells. Normal-growing wild-type, SIN1−/− or HA–SIN1-reconstituted SIN1−/− MEFs (A) or rictor−/− MEFs (B) were either untreated (0) or treated with 17-AAG (1 μM) for 4 or 8 h before harvest, followed by immunoblotting analysis for Akt, PKCα and PKCθ levels. ERK2 and tubulin expression was assessed as a loading control. (C) Inhibition of Hsp90 robustly augmented Akt ubiquitination in SIN1−/− cells. Wild-type and SIN1−/− MEFs were co-transfected and treated as in 3B with the addition of 17-AAG (1 μM), alone or with MG132 for another 6 h before harvesting. HA–Akt was immunoprecipitated and bound ubiquitin was detected as in 3B. (D) Heat shock destabilizes Akt and PKCα more pronouncedly in SIN1−/− cells than in wild-type cells. Wild-type and SIN1−/− MEFs, grown at 37 °C, were shifted to a 42 °C incubator for the indicated time periods as indicated before harvesting. Protein levels were determined by immunoblotting. All lanes from each antibody-blotted panel come from the same blot. (E) Combined inhibition of mTOR and Hsp90 diminishes Akt and PKCα protein levels. Wild-type cells were transfected with vector (control) or mTOR siRNA. Two days after transfection, some of the samples were additionally treated with rapamycin (100 nM) for 36 h, followed by treatment with 17-AAG (1 μM) for 4 or 8 h. Total protein levels were determined by immunoblotting.
Figure 5
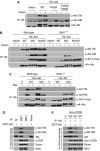
The TM, but not HM, phosphorylation is essential for protein stability of Akt and PKCα. (A) Mutation of the TM, but not the HM, phosphorylation site destabilizes Akt. Wild-type MEFs were stably infected with retroviral expression vectors for HA-tagged wild-type Akt (WT) or mutant Akt at the HM site (S473A), or TM sites (T450A and T443A/T450A). Cells were either untreated (0) or treated with 1 μM 17-AAG for 4 or 8 h before harvest, and the protein levels of HA–Akt were determined by immunoblotting. Infection efficiency was normalized by analysing expression of GFP, which is co-expressed with the genes of interest in an IRES expression cassette (pMIGW). (B) Mutation of the TM, but not HM, phosphorylation site augments Akt ubiquitination. Wild-type or SIN1−/− MEFs that were stably infected with either wild-type HA–Akt (Akt wt) or HA–Akt HM site mutant (Akt S473A) or TM site mutants (Akt T450A, Akt T443A/T450A) were transfected with myc-ubiquitin. Cells were treated and processed as in 3B. (C, D) The absence of TM phosphorylation increases Akt binding to Hsp90. Cellular extracts from wild-type (WT) or SIN1−/− MEFs, both stably infected with HA–Akt (C) and from wild-type MEFs stably infected with HA–Akt wt or TM mutants (D), were subjected to immunoprecipitation using HA antibody. Co-immunoprecipitated Hsp90 were detected by immunoblotting. (E) TM, but not HM, site mutation destabilizes conventional PKCβII. Wild-type or mutant HA–PKC (Thr634/638/641 triple Ala or Ser660Ala) were transfected into HeLa cells. After 36 h, cells were left untreated or treated with 17-AAG for the indicated time (h). Total protein expression was detected by immunoblotting.
Figure 6
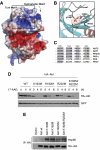
The TM site phosphorylation mediates proper carboxyl-terminal folding and stabilization of Akt structure, by interacting with three conserved basic residues at the catalytic domain. (A) Structural modelling of the TM phosphorylation. Molecular surface of Akt2 (PDB ID: 1O6K), coloured blue (basic) and red (acidic). The TM peptide that is missing in 1O6K (with pT451) was modelled to the structure and energy minimized by simulated annealing with CNS programme (Brunger et al, 1998). pT451 is shown in sticks. Note the top of the N-lobe is highly positively charged, which is ideal for accommodating a phosphorylated threonine. (B) Electrostatic interactions between pT451 from the TM and K165, R184, R224 in the N-lobe. (C) Sequence alignment of residues surrounding K165, R184, R224 and T451 for selected human AGC kinases. Note that T451 is not conserved in PKA and PKCθ, and the R/Ks, particularly R224, are also not conserved in these two enzymes. (D, E) Mutation of each of the conserved basic residues in Akt1 that are predicted to interact with the TM site phosphate led to (D) Akt destabilization upon inhibition of Hsp90 and (E) increased binding of Hsp90. Wild-type MEFs were stably infected with HA–Akt (wild-type or methionine mutants) using retroviral vectors. (D) Before harvest, cells were treated with 17-AAG (1 μM) for the indicated time. Infection efficiency was normalized by analysing expression of GFP (co-expressed under the control of the same promoter). HA–Akt levels were analysed by immunoblotting with HA antibody. (E) Extracts from each cell line were immunoprecipitated using HA antibody. Co-immunoprecipitated Hsp90 was detected by immunoblotting.
Figure 7
Phosphorylation at the TM and HM sites is independently regulated by mTORC2 and requires mTOR kinase activity. (A) The TM site phosphorylation is not a prerequisite for the HM site phosphorylation of Akt. Wild-type MEFs were stably infected with a control vector (Vector) or with retroviral expression vectors for HA–Akt (WT) or mutant (T450A or T443A/T450A) to express comparable and close to physiological levels. Cells were either starved overnight (−), or starved then restimulated with insulin for 30 min before harvest. HA–Akt WT or mutants were immunoprecipitated using an anti-HA antibody, and analysed for phosphorylation and immunoprecipitated levels by immunoblotting. (B) Membrane-targeted Akt is not phosphorylated at the TM site but undergoes partial phosphorylation at the HM site. Wild-type or SIN1−/− MEFS were stably infected with a control vector (Vector) or vectors expressing HA–Akt (WT), myristylated Akt (Myr) or Myr-Akt-kinase dead (MyrKD) as indicated. Cells were starved, stimulated and processed as in A. (C) TM and HM site phosphorylation in wild-type cells is strictly dependent on SIN1/mTORC2. Wild-type and stably infected SIN1−/− MEF cells as described in panel B were treated with rapamycin (100 nM) for 36 h before processing as described in panel A. (D) A SIN1-associated kinase phosphorylates Akt at HM and TM sites in vitro. HeLa cells were immunoprecipitated with control Ig or SIN1 antibody. Immunoprecipitates were used to phosphorylate GST–Akt in vitro. Phosphorylation of GST-Akt and amounts of immunoprecipitated mTORC2 components were analysed by immunoblotting. (E) mTOR kinase activity is required for Akt phosphorylation at HM and TM sites. HeLa cells were transfected with a control vector (V) or with expression vector for HA-tagged mTOR (WT) or a kinase-dead mutant form of HA–mTOR (KD). HA–mTOR (WT and KD) was immunoprecipitated with an anti-HA antibody and used to phosphorylate GST–Akt in vitro. Phosphorylation of Akt and amounts of immunoprecipitated mTORC2 components were analysed by immunoblotting.
Figure 8
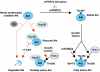
Model for Akt regulation by mTORC2. mTORC2 phosphorylates newly synthesized Akt at the TM (Thr450) site to facilitate carboxyl-terminal folding and to stabilize Akt. In the presence of growth factors, Akt is further phosphorylated at the HM (Ser473) and A-loop (Thr308) sites by mTORC2 and PDK1, respectively, leading to full activation of Akt. When mTORC2 is disrupted genetically or by prolonged rapamycin treatment, Akt is unstable due to lack of TM site phosphorylation. However, in the absence of TM phosphorylation, Akt becomes protected by Hsp90 from ubiquitination-mediated proteasome degradation. Rapid degradation of Akt occurs upon simultaneous inhibition of mTORC2 and Hsp90.
Similar articles
- Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling.
Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Ikenoue T, et al. EMBO J. 2008 Jul 23;27(14):1919-31. doi: 10.1038/emboj.2008.119. Epub 2008 Jun 19. EMBO J. 2008. PMID: 18566587 Free PMC article. - SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity.
Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. Jacinto E, et al. Cell. 2006 Oct 6;127(1):125-37. doi: 10.1016/j.cell.2006.08.033. Epub 2006 Sep 7. Cell. 2006. PMID: 16962653 - Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity.
Yang Q, Inoki K, Ikenoue T, Guan KL. Yang Q, et al. Genes Dev. 2006 Oct 15;20(20):2820-32. doi: 10.1101/gad.1461206. Genes Dev. 2006. PMID: 17043309 Free PMC article. - mTOR Regulation of AGC Kinases: New Twist to an Old Tail.
Baffi TR, Newton AC. Baffi TR, et al. Mol Pharmacol. 2022 Apr;101(4):213-218. doi: 10.1124/molpharm.121.000310. Epub 2021 Jun 21. Mol Pharmacol. 2022. PMID: 34155089 Free PMC article. Review. - TOR regulation of AGC kinases in yeast and mammals.
Jacinto E, Lorberg A. Jacinto E, et al. Biochem J. 2008 Feb 15;410(1):19-37. doi: 10.1042/BJ20071518. Biochem J. 2008. PMID: 18215152 Review.
Cited by
- Emerging role of mTOR in the response to cancer therapeutics.
Ilagan E, Manning BD. Ilagan E, et al. Trends Cancer. 2016 May;2(5):241-251. doi: 10.1016/j.trecan.2016.03.008. Trends Cancer. 2016. PMID: 27668290 Free PMC article. - mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis.
Chou SD, Prince T, Gong J, Calderwood SK. Chou SD, et al. PLoS One. 2012;7(6):e39679. doi: 10.1371/journal.pone.0039679. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768106 Free PMC article. - Dual targeting of eIF4E by blocking MNK and mTOR pathways in leukemia.
Kosciuczuk EM, Saleiro D, Platanias LC. Kosciuczuk EM, et al. Cytokine. 2017 Jan;89:116-121. doi: 10.1016/j.cyto.2016.01.024. Epub 2016 Apr 16. Cytokine. 2017. PMID: 27094611 Free PMC article. Review. - Live and let die: signaling AKTivation and UPRegulation dynamics in SARS-CoVs infection and cancer.
Suaya M, Sánchez GM, Vila A, Amante A, Cotarelo M, García Carrillo M, Blaustein M. Suaya M, et al. Cell Death Dis. 2022 Oct 3;13(10):846. doi: 10.1038/s41419-022-05250-5. Cell Death Dis. 2022. PMID: 36192392 Free PMC article. Review. - Coordinate phosphorylation of multiple residues on single AKT1 and AKT2 molecules.
Guo H, Gao M, Lu Y, Liang J, Lorenzi PL, Bai S, Hawke DH, Li J, Dogruluk T, Scott KL, Jonasch E, Mills GB, Ding Z. Guo H, et al. Oncogene. 2014 Jun 26;33(26):3463-72. doi: 10.1038/onc.2013.301. Epub 2013 Aug 5. Oncogene. 2014. PMID: 23912456 Free PMC article.
References
- Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P (1998) Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17: 313–325 - PubMed
- Biondi RM (2004) Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem Sci 29: 136–142 - PubMed
- Bornancin F, Parker PJ (1996) Phosphorylation of threonine 638 critically controls the dephosphorylation and inactivation of protein kinase Calpha. Curr Biol 6: 1114–1123 - PubMed
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL070225/HL/NHLBI NIH HHS/United States
- HL070225/HL/NHLBI NIH HHS/United States
- U19 DK062434/DK/NIDDK NIH HHS/United States
- DK62434/DK/NIDDK NIH HHS/United States
- R01 GM079176/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous