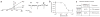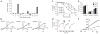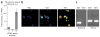Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels - PubMed (original) (raw)
Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels
Diana M Bautista et al. Nat Neurosci. 2008 Jul.
Abstract
In traditional folk medicine, Xanthoxylum plants are referred to as 'toothache trees' because their anesthetic or counter-irritant properties render them useful in the treatment of pain. Psychophysical studies have identified hydroxy-alpha-sanshool as the compound most responsible for the unique tingling and buzzing sensations produced by Szechuan peppercorns or other Xanthoxylum preparations. Although it is generally agreed that sanshool elicits its effects by activating somatosensory neurons, the underlying cellular and molecular mechanisms remain a matter of debate. Here we show that hydroxy-alpha-sanshool excites two types of sensory neurons, including small-diameter unmyelinated cells that respond to capsaicin (but not mustard oil) as well as large-diameter myelinated neurons that express the neurotrophin receptor TrkC. We found that hydroxy-alpha-sanshool excites neurons through a unique mechanism involving inhibition of pH- and anesthetic-sensitive two-pore potassium channels (KCNK3, KCNK9 and KCNK18), providing a framework for understanding the unique and complex psychophysical sensations associated with the Szechuan pepper experience.
Figures
Figure 1
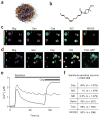
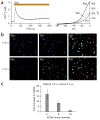
Hydroxy-α-sanshool excites a subset of presumptive nociceptors and mechanoreceptors. (a) Szechuan peppers are the spicy berries of Xanthoxylum piperitum, a species of prickly ash found in China and Japan. (b) Structure of hydroxy-α-sanshool, the main pungent compound from Xanthoxylum plants. (c) Cultured sensory neurons were exposed to sanshool (San; 100 μM) followed by capsaicin (Cap; 1 μM) and subsequently 140 mM potassium chloride (KCl) and were analyzed by calcium imaging. No response to sanshool was observed in the absence of extracellular calcium, demonstrating that the calcium signal is due to influx (data not shown). After calcium imaging, neurons were fixed and probed for NF200 reactivity by immunohistochemistry. Responses to sanshool were observed in NF200- positive, capsaicin-insensitive neurons (cell 1) as well as NF200-negative, capsaicin-sensitive neurons (cell 2). (d) Cultured DRG sensory neurons from TrkC-GFP mice were treated and analyzed as in c. Sanshool responses were observed in TrkC-positive, capsaicin-insensitive neurons (cell 1) as well as TrkC-negative, capsaicin-sensitive neurons (cell 2). (e) Calcium imaging shows that some sanshool-sensitive cells are capsaicin sensitive, whereas others are not, corresponding to small- and large-diameter neurons, respectively (average sizes = 18.0 and 35.7 μm; average response from 15 representative cells). (f) Quantitative analysis of concordance between sanshool sensitivity and other histological or pharmacological attributes. Cells showing sensitivity to sanshool (100 μM) were examined for activation by capsaicin (Cap; 1 μM), mustard oil (MO; 100 μM), menthol (ME; 500 μM) or hypo-osmotic (Osmo; 226 mOsm) stimuli, as well as for expression of TrkC or neurofilament (NF200) immunoreactivity. Note the high (>40%) preponderance of capsaicin or hypo-osmotic sensitivity among sanshoolsensitive neurons, as compared to the relatively low (≤1%) sensitivity to mustard oil or menthol. Moreover, many (≥50%) of sanshool-positive cells were myelinated (NF200 positive) and/or TrkC positive.
Figure 2
Sanshool inhibits pH-sensitive background potassium channels in sensory neurons. (a) Representative whole-cell voltage-clamp recording from a cultured trigeminal neuron subjected to a voltage ramp (+60 mV to −100 mV, 100 ms; applied every 2 s). Current-voltage relationship before (Bkg) or after application of sanshool (100 μM) or low pH (pH 6.5) (left). Average current recorded at −60 mV in response to sanshool or low pH (right). (b) Dose-response curve of sanshool-evoked inhibition of background potassium conductance in sensory neurons (holding potential = –60 mV) recorded in extracellular Ringer’s solution (IC50 = 69.5 ± 5.3 μM; n = 3–7 cells per point). (c) Summary of sanshool-sensitive currents measured in small- and large-diameter neurons.
Figure 3
Sanshool inhibits KCNK3, KCNK9 and KCNK18. (a) Xenopus oocytes expressing a given KCNK family member were subjected to two-electrode voltage-clamp analysis and the percent suppression of leak current was determined after bath application of purified sanshool (100 μM) (n = 5–8 cells per channel). (b) Representative traces of sanshool-evoked inhibition of KCNK3 (left), KCNK9 (middle) and KCNK18 (right) in oocytes (holding potential = −80 mV). Inhibition by extracellular protons (pH 6.5) and barium (2 mM) is shown for comparison. (c) Dose-response curves of sanshool-evoked inhibition at 0 mV of KCNK3, KCNK9, KCNK18 or KCNK3/KCNK9 heteromers, recorded in Xenopus oocytes (n = 5–8 cells per point). (d) Representative current recorded from Xenopus oocytes expressing KCNK18 in response to sanshool (100 μM) or low pH (pH 6.5) (holding potential = −60mV, n = 5). (e) Sanshool-evoked inhibition of KCNK channels at pH 6.5, pH 7.4 and pH 8.5 (n = 4–10 oocytes per condition). (f) Inhibition of KCNK18 currents by sanshool (100 μM) applied to an inside-out patch from transfected HEK293 cells (n = 7). Seals were obtained with extracellular Ringer’s solution in both pipette and bath. After excision of the patch, bath solution was replaced with high-potassium Ringer’s (see Methods). Sanshool has no effect on background currents observed in vector-transfected control cells (not shown).
Figure 4
TRPA1 and TRPV1 are not required for sanshool sensitivity. (a) Sanshool elicits small, but detectable, currents in TRPV1- (left) or TRPA1-expressing (middle) Xenopus oocytes but only at positive membrane potentials. Bar graph (right) shows summary of sanshool (1 mM)-evoked currents recorded at +60 versus −60 mV holding potential (n = 5). (b) Sanshool-evoked calcium influx in trigeminal sensory neurons is not blocked by ruthenium red (10 μM), a blocker of TRPV1 and TRPA1 channels (n = 114). (c) Sanshool-evoked calcium influx is normal in neurons cultured from mice deficient in both TRPV1 and TRPA1 (n = 108). (d) Consumption of water containing sanshool (1 mM) was significantly decreased in TRPA1 TRPV1–deficient mice (closed circles), as well as in their wild-type littermates (open circles). In contrast, only wild-type animals showed decreased consumption of water containing capsaicin and mustard oil. **P o 0.01, one-way ANOVA; n = 10 animals per genotype.
Figure 5
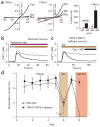
Sanshool excites CNS neurons that express KCNK3, KCNK9 or KCNK18. (a) Representative calcium response of cultured cerebellar granule neurons (CGNs) in response to sanshool (100 μM; left). Representative electrophysiological response of a CGN to sanshool (100 μM) or protons (pH 6.5) during whole-cell voltage-clamp recording (holding potential = −60 mV) (right). (b) Comparison of sanshool-evoked calcium responses by CGNs cultured for 2 d (top) versus 7 d (bottom) (days in vitro, d.i.v.). (c) Quantitative PCR analysis of sanshool-sensitive KCNK transcripts from CGN cultured for 7 d.i.v. versus 2 (n = 3–4).
Figure 6
Sanshool excites sensory neurons that express KCNK3, KCNK9 or KCNK18. (a) Quantitative PCR analysis of sanshoolsensitive KCNK expression in cultured trigeminal (TG) sensory neurons versus CGN cultured for 2 d.i.v. (n = 3–4). (b) Representative calcium imaging experiment used to identify sanshoolsensitive cells. Cells 1 and 2 are sanshoolsensitive, whereas cells 3 and 4 are insensitive. (c) Representative PCR analysis of KCNK18 expression in sanshool-positive and sanshoolnegative sensory neurons. Lane 1 contains a sample amplified from cDNA prepared from cells 1 and 2; lane 2 contains a sample amplified from cDNA prepared from cells 3 and 4 (see above). n = 6 samples for sanshool-sensitive neurons and 3 for sanshool-insensitive neurons; each sample contained 2–3 cells. Note the presence of control RPL19 product in all samples (right).
Similar articles
- Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons.
Koo JY, Jang Y, Cho H, Lee CH, Jang KH, Chang YH, Shin J, Oh U. Koo JY, et al. Eur J Neurosci. 2007 Sep;26(5):1139-47. doi: 10.1111/j.1460-9568.2007.05743.x. Eur J Neurosci. 2007. PMID: 17767493 - A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats.
Klein AH, Sawyer CM, Zanotto KL, Ivanov MA, Cheung S, Carstens MI, Furrer S, Simons CT, Slack JP, Carstens E. Klein AH, et al. J Neurophysiol. 2011 Apr;105(4):1701-10. doi: 10.1152/jn.00922.2010. Epub 2011 Jan 27. J Neurophysiol. 2011. PMID: 21273322 Free PMC article. - Transient receptor potential channels in sensory neurons are targets of the antimycotic agent clotrimazole.
Meseguer V, Karashima Y, Talavera K, D'Hoedt D, Donovan-Rodríguez T, Viana F, Nilius B, Voets T. Meseguer V, et al. J Neurosci. 2008 Jan 16;28(3):576-86. doi: 10.1523/JNEUROSCI.4772-07.2008. J Neurosci. 2008. PMID: 18199759 Free PMC article. - Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing.
Nagata K, Duggan A, Kumar G, García-Añoveros J. Nagata K, et al. J Neurosci. 2005 Apr 20;25(16):4052-61. doi: 10.1523/JNEUROSCI.0013-05.2005. J Neurosci. 2005. PMID: 15843607 Free PMC article. - Molecular and cellular mechanisms of trigeminal chemosensation.
Gerhold KA, Bautista DM. Gerhold KA, et al. Ann N Y Acad Sci. 2009 Jul;1170:184-9. doi: 10.1111/j.1749-6632.2009.03895.x. Ann N Y Acad Sci. 2009. PMID: 19686135 Free PMC article. Review.
Cited by
- The family of K2P channels: salient structural and functional properties.
Feliciangeli S, Chatelain FC, Bichet D, Lesage F. Feliciangeli S, et al. J Physiol. 2015 Jun 15;593(12):2587-603. doi: 10.1113/jphysiol.2014.287268. Epub 2015 Jan 22. J Physiol. 2015. PMID: 25530075 Free PMC article. Review. - Touch sense: functional organization and molecular determinants of mechanosensitive receptors.
Roudaut Y, Lonigro A, Coste B, Hao J, Delmas P, Crest M. Roudaut Y, et al. Channels (Austin). 2012 Jul-Aug;6(4):234-45. doi: 10.4161/chan.22213. Channels (Austin). 2012. PMID: 23146937 Free PMC article. Review. - Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation.
Mishra SK, Hoon MA. Mishra SK, et al. Mol Cell Neurosci. 2010 Jan;43(1):157-63. doi: 10.1016/j.mcn.2009.10.006. Epub 2009 Oct 21. Mol Cell Neurosci. 2010. PMID: 19853036 Free PMC article. - Two-pore domain potassium channels: emerging targets for novel analgesic drugs: IUPHAR Review 26.
Gada K, Plant LD. Gada K, et al. Br J Pharmacol. 2019 Jan;176(2):256-266. doi: 10.1111/bph.14518. Epub 2018 Dec 3. Br J Pharmacol. 2019. PMID: 30325008 Free PMC article. Review. - An Exploratory Study on the Contractile Effects of Hydroxy-α-Sanshool and Hydroxy-β-Sanshool, the Active Ingredients of Daikenchuto, on the Internal Anal Sphincter.
Maeda K, Sasaki T. Maeda K, et al. J Anus Rectum Colon. 2023 Jul 25;7(3):206-213. doi: 10.23922/jarc.2023-001. eCollection 2023. J Anus Rectum Colon. 2023. PMID: 37496571 Free PMC article.
References
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Elsevier; Philadelphia: 2006. pp. 3–34.
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. - PubMed
- Fields HL. Pain. McGraw-Hill; New York: 1987.
- Snyder SH. Opiate receptors and internal opiates. Sci Am. 1977;236:44–56. - PubMed
- Julius D. From peppers to peppermints: natural products as probes of the pain pathway. Harvey Lect. 2005;101:89–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases