Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory - PubMed (original) (raw)
doi: 10.1038/nm1782. Epub 2008 Jun 22.
Shaomin Li, Tapan H Mehta, Amaya Garcia-Munoz, Nina E Shepardson, Imelda Smith, Francesca M Brett, Michael A Farrell, Michael J Rowan, Cynthia A Lemere, Ciaran M Regan, Dominic M Walsh, Bernardo L Sabatini, Dennis J Selkoe
Affiliations
- PMID: 18568035
- PMCID: PMC2772133
- DOI: 10.1038/nm1782
Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory
Ganesh M Shankar et al. Nat Med. 2008 Aug.
Abstract
Alzheimer's disease constitutes a rising threat to public health. Despite extensive research in cellular and animal models, identifying the pathogenic agent present in the human brain and showing that it confers key features of Alzheimer's disease has not been achieved. We extracted soluble amyloid-beta protein (Abeta) oligomers directly from the cerebral cortex of subjects with Alzheimer's disease. The oligomers potently inhibited long-term potentiation (LTP), enhanced long-term depression (LTD) and reduced dendritic spine density in normal rodent hippocampus. Soluble Abeta from Alzheimer's disease brain also disrupted the memory of a learned behavior in normal rats. These various effects were specifically attributable to Abeta dimers. Mechanistically, metabotropic glutamate receptors were required for the LTD enhancement, and N-methyl D-aspartate receptors were required for the spine loss. Co-administering antibodies to the Abeta N-terminus prevented the LTP and LTD deficits, whereas antibodies to the midregion or C-terminus were less effective. Insoluble amyloid plaque cores from Alzheimer's disease cortex did not impair LTP unless they were first solubilized to release Abeta dimers, suggesting that plaque cores are largely inactive but sequester Abeta dimers that are synaptotoxic. We conclude that soluble Abeta oligomers extracted from Alzheimer's disease brains potently impair synapse structure and function and that dimers are the smallest synaptotoxic species.
Figures
Figure 1
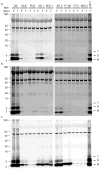
Monomeric and oligomeric Aβ is detected in brain extracts of humans with clinically and neuropathologically typical late-onset AD. IP/WB analysis (see Methods) was performed on supernatants of the soluble (a, TBS), membrane-associated (b, TBS-TX100) and insoluble (c, GuHCl) sequential extracts of frontal (F) and temporal (T) cortex homogenates from various individuals diagnosed with different forms of dementia (see Supp. Table 1). Samples were IP’ed with polyclonal Aβ antibody R1282 and blotted with monoclonals 2G3 (Aβ40) + 21F12 (Aβ42). Subject key: DS, Down’s syndrome with AD; DLB, dementia with Lewy bodies; PCD, paraneoplastic cerebellar degeneration; AD, Alzheimer’s disease; MID, multi-infarct dementia; P-AD, “pathological AD” (i.e., scattered amyloid plaques without a history of clinical AD); FTD, frontotemporal dementia. See Supp. Table 1a for further information.
Figure 2
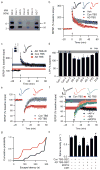
Soluble Aβ extracted from AD brain alters hippocampal synapse physiology and learned behavior. (a) IP/WB of the TBS brain extracts used to study LTP. Clinical and neuropathological diagnoses for each of these seven cases are provided in Supp. Table 1b. (b) Summary data of LTP induction following two 100 Hz stimuli (HFS, arrow) of slices treated with 500 μL each of TBS vehicle (Veh; _n_=8 slices), Con 1 TBS extract (Con TBS; _n_=6) or AD 4 TBS extract (AD TBS; _n_=8). Insets show average baseline (light) and post-HFS (dark) fEPSP traces; calibration bars 5 msec/0.2 mV. (c) LTP is induced normally in hippocampal slices treated with 500 μL of immunodepleted AD TBS (AD TBS-ID). The summary LTP data for Con TBS (blue, _n_=6 slices) and AD TBS (red, _n_=8 slices) from Fig. 2b are represented as horizontal bars depicting means ± SEMs of fEPSP slopes at ~50–60 min post-HFS. Inset: Two sequential IP’s (R1282) of AD TBS blotted with 2G3+21F12. (d) Summary LTP data (means ± SEMs) for 3 different control subjects (Con 1, _n_=6; Con 2, _n_=6; Con 3, _n_=6), 4 AD subjects (AD 4 _n_=8; AD 5, _n_=5; AD 6, _n_=6; AD 7, _n_=6), 1 FTD subject (_n_=5) and 1 DLB subject (_n_=6). For comparison, LTP data from Fig. 2b for Veh are represented by the grey horizontal bar. *P<0.05 compared with Veh. (e) Summary LTD data for slices treated with 500 μL Con TBS (blue, _n_=7) or AD TBS (red, _n_=8) for 10 min prior to a weak stimulation protocol of 300 pulses at 1 Hz, indicated by the small grey bar. Calibration bars 5 msec/0.2 mV. (f) Summary LTD data for co-administration of AD TBS with either 50 μM AP-V (black, _n_=8), 500 μM (R/S)-MCPG (green, _n_=7) or 3 μM SIB1757 (orange, _n_=6). For comparison, LTD data (means ± SEM) for Con TBS (blue) and AD TBS (red) are shown as horizontal bars. (g) Cumulative probability distribution representing escape latency for animals receiving AD TBS (red) or AD TBS-ID (black) at 48 hr after training. Animals receiving AD TBS had a significantly shorter mean escape latency than animals receiving AD TBS-ID (174 ± 31.7 sec and 255 ± 22.8 sec, respectively; P<0.05; _n_=11 and 10 rats). h. Summary spine density data for pyramidal cells exposed to SEC-enriched TBS extract from AD brain (AD TBS-SEC) or from Con brain (Con TBS-SEC) and also for slices treated with 500 μM MCPG or 20 μM CPP in the presence or absence of AD TBS-SEC. *,#P<0.05 vs. Con TBS-SEC and vs. AD TBS-SEC, respectively.
Figure 3
Soluble dimers are the smallest Aβ assembly form in human brain to acutely perturb synapse physiology. (a) TBS extracts of AD (top panel) and control (bottom panel) brains were subjected to non-denaturing SEC. SEC fractions were lyophilized and WB’d with 2G3+21F12; molecular weights (in kDa) on left. Note that Aβ monomer and dimer in the AD TBS extract is recovered from material that elutes at the end of the void volume (fractions 3/4). Control (Con) brain extracts were devoid of Aβ. (b) Summary LTP data (means ± SEMs) for slices treated with SEC fractions from AD or Con TBS extracts (_n_=6 slices for all samples), as characterized in Fig. 3a. (c) Representative WB (2G3+21F12) of IP-SEC fractionation of AD TBS and Con TBS. TBS extracts (500 μL) were immunoprecipitated with 3D6 (3 μg/mL), eluted with sample buffer and subjected to SEC. Dimer-enriched (fractions 7–8) and monomer-enriched (fractions 10–11) IP-SEC fractions were separately pooled, as were corresponding fractions from Con TBS. (d) Summary LTP data (means ± SEMs) for slices treated with IP-SEC fractions of AD and control brain TBS extracts, as characterized in Fig. 3c (Con fractions 10–11, _n_=5; AD fractions 10–11, _n_=5; Con fractions 7–8, _n_=7; AD fractions 7–8, _n_=7). (e) Mutant Aβ40-S26C forms dimers under oxidizing conditions (ox), which can be reduced to monomers by treating with β-ME (red). Silver stain was performed with 100 ng wildtype Aβ40 (wt) peptide or the mutant peptide (f) Summary LTP data (means ± SEMs) for slices treated with 5, 50, or 100 nM of either wt Aβ40 (black) or oxidized Aβ40-S26C (red) reveals that the oxidized Aβ40-S26C dimer inhibits LTP with much greater potency (100 nM Aβ40-S26C, _n_=4; _n_=5 for all other treatments). The vehicle controls (plotted at 0 nM) were 50 mM ammonium acetate (_n_=4) for the S26C peptide and 0.1% ammonium hydroxide (_n_=4) for wt peptide.
Figure 4
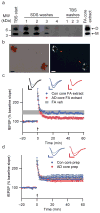
Insoluble amyloid cores contain Aβ dimers with synaptotoxic potential but are not readily released. (a) IP/WB of sequential extracts of the TBS-insoluble pellet prepared from 100 mg of a plaque-rich AD brain (case AD 5 from Fig. 2a) (see Methods). The final TBS washes reveal that no additional soluble Aβ can be extracted from the pellet after 4 sequential SDS washes. The remaining core-rich pellet was then incubated in formic acid (FA core extract) and analyzed by IP/WB, revealing that the insoluble cores contain Aβ monomers and dimers (far right lane). (b) Core preps following the final TBS wash as in Fig. 4a were stained with 0.2% Congo red and visualized by brightfield (left) and polarization (right) microscopy. Isolated amyloid cores display characteristic birefringence with Congo red (red arrowheads). Material prepared similarly from Con 3 (Fig. 2a) did not contain any such structures. Scale bar = 5 μm. (c) Cores prepared as in Figs. 4a, b were extracted with 88% formic acid and neutralized with NaOH. Summary LTP data for slices treated with just FA/NaOH vehicle (FA Veh, _n_=5), or with FA/NaOH core extracts from AD (AD core FA extract, _n_=7) or control (Con core FA extract, _n_=5) brains. Calibration bars, 5 msec/0.2 mV. (d) Summary LTP data for slices exposed to intact core preps isolated as in Fig. 4a, b from 100 mg AD cortex (AD core prep, _n_=5) or Con cortex (Con core prep, _n_=5). Calibration bars, 5 msec/0.3 mV.
Similar articles
- Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers.
Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Laurén J, et al. Nature. 2009 Feb 26;457(7233):1128-32. doi: 10.1038/nature07761. Nature. 2009. PMID: 19242475 Free PMC article. - Tau and Amyloid β Protein in Patient-Derived Aqueous Brain Extracts Act Concomitantly to Disrupt Long-Term Potentiation in Vivo.
Ondrejcak T, Klyubin I, Hu NW, O'Malley TT, Corbett GT, Winters R, Perkinton MS, Billinton A, Prenderville JA, Walsh DM, Rowan MJ. Ondrejcak T, et al. J Neurosci. 2023 Aug 9;43(32):5870-5879. doi: 10.1523/JNEUROSCI.0082-23.2023. Epub 2023 Jul 25. J Neurosci. 2023. PMID: 37491315 Free PMC article. - Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior.
Selkoe DJ. Selkoe DJ. Behav Brain Res. 2008 Sep 1;192(1):106-13. doi: 10.1016/j.bbr.2008.02.016. Epub 2008 Feb 17. Behav Brain Res. 2008. PMID: 18359102 Free PMC article. Review. - Large Soluble Oligomers of Amyloid β-Protein from Alzheimer Brain Are Far Less Neuroactive Than the Smaller Oligomers to Which They Dissociate.
Yang T, Li S, Xu H, Walsh DM, Selkoe DJ. Yang T, et al. J Neurosci. 2017 Jan 4;37(1):152-163. doi: 10.1523/JNEUROSCI.1698-16.2016. J Neurosci. 2017. PMID: 28053038 Free PMC article. - Synaptic changes in Alzheimer's disease and its models.
Pozueta J, Lefort R, Shelanski ML. Pozueta J, et al. Neuroscience. 2013 Oct 22;251:51-65. doi: 10.1016/j.neuroscience.2012.05.050. Epub 2012 Jun 9. Neuroscience. 2013. PMID: 22687952 Review.
Cited by
- Cytotoxic Effect of Amyloid-β1-42 Oligomers on Endoplasmic Reticulum and Golgi Apparatus Arrangement in SH-SY5Y Neuroblastoma Cells.
Jarero-Basulto JJ, Gasca-Martínez Y, Rivera-Cervantes MC, Gasca-Martínez D, Carrillo-González NJ, Beas-Zárate C, Gudiño-Cabrera G. Jarero-Basulto JJ, et al. NeuroSci. 2024 May 7;5(2):141-157. doi: 10.3390/neurosci5020010. eCollection 2024 Jun. NeuroSci. 2024. PMID: 39483494 Free PMC article. - The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology.
Fanlo-Ucar H, Picón-Pagès P, Herrera-Fernández V, Ill-Raga G, Muñoz FJ. Fanlo-Ucar H, et al. Antioxidants (Basel). 2024 Oct 8;13(10):1208. doi: 10.3390/antiox13101208. Antioxidants (Basel). 2024. PMID: 39456461 Free PMC article. Review. - Morphological and Molecular Profiling of Amyloid-β Species in Alzheimer's Pathogenesis.
Almeida ZL, Vaz DC, Brito RMM. Almeida ZL, et al. Mol Neurobiol. 2024 Oct 24. doi: 10.1007/s12035-024-04543-4. Online ahead of print. Mol Neurobiol. 2024. PMID: 39446217 Review. - Potential Mechanisms of Tunneling Nanotube Formation and Their Role in Pathology Spread in Alzheimer's Disease and Other Proteinopathies.
Kotarba S, Kozłowska M, Scios M, Saramowicz K, Barczuk J, Granek Z, Siwecka N, Wiese W, Golberg M, Galita G, Sychowski G, Majsterek I, Rozpędek-Kamińska W. Kotarba S, et al. Int J Mol Sci. 2024 Oct 8;25(19):10797. doi: 10.3390/ijms251910797. Int J Mol Sci. 2024. PMID: 39409126 Free PMC article. Review. - β-Amyloids and Immune Responses Associated with Alzheimer's Disease.
Kolobova E, Petrushanko I, Mitkevich V, Makarov AA, Grigorova IL. Kolobova E, et al. Cells. 2024 Sep 28;13(19):1624. doi: 10.3390/cells13191624. Cells. 2024. PMID: 39404388 Free PMC article. Review.
References
- Morgan D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–5. - PubMed
- Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG027443-03/AG/NIA NIH HHS/United States
- AG R01 027443/AG/NIA NIH HHS/United States
- 067660/WT_/Wellcome Trust/United Kingdom
- R01 NS046579-06A1/NS/NINDS NIH HHS/United States
- R01 AG027443-01/AG/NIA NIH HHS/United States
- R01 AG027443/AG/NIA NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 AG027443-04/AG/NIA NIH HHS/United States
- R01 AG027443-02/AG/NIA NIH HHS/United States
- R01 NS046579/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases