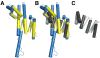Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex - PubMed (original) (raw)
Structural and functional studies of Nup107/Nup133 interaction and its implications for the architecture of the nuclear pore complex
Thomas Boehmer et al. Mol Cell. 2008.
Abstract
Nuclear pore complexes (NPCs) are 40-60 MDa protein assemblies embedded in the nuclear envelope of eukaryotic cells. NPCs exclusively mediate all transport between cytoplasm and nucleus. The nucleoporins that build the NPC are arranged in a stable core of module-like subcomplexes with eight-fold rotational symmetry. To gain insight into the intricate assembly of the NPC, we have solved the crystal structure of a protein complex between two nucleoporins, human Nup107 and Nup133. Both proteins form elongated structures that interact tightly via a compact interface in tail-to-tail fashion. Additional experiments using structure-guided mutants show that Nup107 is the critical anchor for Nup133 to the NPC, positioning Nup133 at the periphery of the NPC. The significant topological differences between Nup107 and Nup133 suggest that *-helical nucleoporin domains of the NPC scaffold fall in different classes and fulfill largely nonredundant functions.
Figures
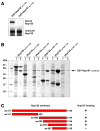
Figure 1. Nup107 and Nup133 Interact in Tail-to-Tail Fashion
(A) In vitro binding of full length human Nup133 to recombinant GST-tagged human Nup107 visualized by autoradiography. Top and bottom panels show the bound and unbound fractions of [35S] methionine-labeled Nup133 translation product incubated with recombinant GST-Nup107 proteins immobilized on affinity resin. The C-terminus of Nup107 is critical for its interaction with Nup133. (B) Various C-terminal fragments of Nup133 (marked with asterisks) were co-expressed with GST-tagged Nup107 (aa 658–925) from separate vectors in E. coli and co-purified by glutathione sepharose affinity chromatography. Total cell lysates (t) and elutions from the GST-affinity columns (e) were separated by SDS-PAGE and analyzed by Coomassie staining. (C) Schematic representation of Nup133 constructs tested for Nup107-binding (+). A construct spanning residues 934 to 1156 of Nup133 represents the minimum domain required for the interaction with Nup107. The relative amounts of co-expressed proteins differs from clone to clone, due to pGEX- and pET-derived vectors harboring the same origin of replication. The results of the binding experiment are therefore limited to qualitative interpretation.
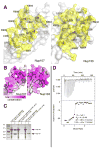
Figure 2. The Nup107/Nup133 Complex Structure
(A) Overall structure of the Nup107658–925/Nup133934–1156 interaction complex. Nup107 is colored in blue, Nup133 in orange. Both proteins are entirely α-helical and interact via a compact interface, mainly involving two helices from each binding partner. Secondary structure elements in Nup107 are labeled (helices α4 and α8 of Nup107 are obscured and labels omitted for clarity). Nup107 forms an irregular stack of helices, with helix 6 protruding noticeably from the domain core. (B) Overall structure rotated by 90° compared to (A). Helices in Nup133 are numbered. Nup133 consists of two connected domains, an interacting 4-helix bundle and the C-terminal domain. (C) Schematic domain organization of Nup107 and Nup133. Important residue positions are indicated. α-helical domains are marked in grey outside of the crystallized regions which are colored in blue and orange, respectively. The β-propeller domain (PDB code 1XKS) is colored white. (D) Superposition of the Nup107658–925/Nup133934–1156 interaction complex crystallized in two different crystal forms, shown as tubes. In the protein construct used in crystal form 2 the helix 6 protrusion is replaced by a short flexible linker. Tube thickness proportional to temperature factors, indicating flexibility. The C-terminal helical domain of Nup133 is significantly more flexible than the rest of the protein and shows rigid body movement.
Figure 3. The Nup107/Nup133 Interaction
(A) Open book view of the Nup107/Nup133 interface, Nup107 contact side shown in the left panel, Nup133 contact side in the right panel. Protein surface is shown half-transparent, contact region colored yellow. The contact area measures 1180 Å2. Interface residues are labeled. The interface is ‘dry’ and entirely hydrophobic in its core. (B) Conservation of the binding interface across eukaryotes. (C) Structure-based mutants disrupt the interaction between Nup107 and Nup133. Wildtype and mutant Nup133 (aa 517–1156) was co-expressed with wildtype and mutant GST-tagged Nup107 (aa 658–925) from separate vectors in E. coli and co-purified by glutathione sepharose affinity chromatography. Total cell lysates (t) and elutions from the GST-affinity columns (e) were separated by SDS-PAGE and analyzed by Coomassie staining. Both double mutations, Nup107 (A878E L901E) and Nup133 (L973E L976E), are sufficient to abolish the interaction of Nup107 and Nup133. Regarding relative amount of expressed proteins see comment in legend to Figure 1. (D) Isothermal titration calorimetry. The titration curve of Nup107658–925 against Nup133934–1156 demonstrates a binding stoichiometry of 1.0 ± 0.1. 8 μl aliquots of Nup107 (13.1μM) were successively injected into 1.38 ml of a 0.75 μM solution of Nup133. The dissociation constant KD, binding enthalpy ΔH and entropy TΔS were derived by curve fitting using the single set of independent sites model. The binding reaction is enthalpically driven.
Figure 4. Comparison to α-Helical Repeat Proteins
Superposition of Nup107 (panel A) and the consensus α-helical TPR-repeat protein (PDB code 2FO7) (panel C) is shown in (B). RMS deviation 4.0 Å over 92 equivalent Cα positions (indicated in yellow). The similarity between Nup107 and αhelical repeat proteins is limited, in contrast to current computer predictions. No significant match with α-helical repeats was found for Nup133 (not shown).
Figure 5. Nup107 and Nup133 are Anchored to the NPC via the N-terminus of Nup107
(A) Localization of EGFP-tagged Nup133 proteins in HeLa cells. Cells, co-stained with the NPC marker mAb414, were analyzed 48 h after transfection. In contrast to full length Nup133 (A–C), showing punctate nuclear rim staining, the non-Nup107-binding mutant Nup133 (L973E L976E) is dispersed over the cytoplasm and largely excluded from the nucleus and nuclear envelope (D–F). (B) Localization of EGFP-tagged Nup107 proteins in HeLa cells. Cells, co-stained with mAb414, were analyzed 48 h after transfection. EGFP-tagged Nup107 lacking the last ~250 residues shows punctate nuclear rim staining that overlaps with the mAb414 signal (D–F), similar to full length Nup107 (A–C). In contrast, the Nup133-binding C-terminus of Nup107 shows a dispersed EGFP-signal, with only a slight concentration at the nuclear envelope (G-J).
Figure 6. The Nup107 α6-Finger is Absent in Budding Yeast
Alignment of the Nup107 amino acid sequence folding into the protruding helix α6 ‘finger’. Sequences representative of the entire phylogenetic tree of eukaryotes are shown. The finger is present in metazoa, but noticeably absent in all budding yeasts analyzed. Maximally diverged budding yeast species are shown, indicating that the finger was lost early after this clade split off from its last common ancestor with other eukaryotes ~300–400 Mya (Dujon, 2006).
Similar articles
- Structural and functional analysis of Nup133 domains reveals modular building blocks of the nuclear pore complex.
Berke IC, Boehmer T, Blobel G, Schwartz TU. Berke IC, et al. J Cell Biol. 2004 Nov 22;167(4):591-7. doi: 10.1083/jcb.200408109. J Cell Biol. 2004. PMID: 15557116 Free PMC article. - Roles of Nup133, Nup153 and membrane fenestrations in assembly of the nuclear pore complex at the end of mitosis.
Bilir Ş, Kojidani T, Mori C, Osakada H, Kobayashi S, Koujin T, Hiraoka Y, Haraguchi T. Bilir Ş, et al. Genes Cells. 2019 May;24(5):338-353. doi: 10.1111/gtc.12677. Epub 2019 Mar 22. Genes Cells. 2019. PMID: 30821042 - Architecture of the linker-scaffold in the nuclear pore.
Petrovic S, Samanta D, Perriches T, Bley CJ, Thierbach K, Brown B, Nie S, Mobbs GW, Stevens TA, Liu X, Tomaleri GP, Schaus L, Hoelz A. Petrovic S, et al. Science. 2022 Jun 10;376(6598):eabm9798. doi: 10.1126/science.abm9798. Epub 2022 Jun 10. Science. 2022. PMID: 35679425 Free PMC article. - Structure and Assembly of the Nuclear Pore Complex.
Hampoelz B, Andres-Pons A, Kastritis P, Beck M. Hampoelz B, et al. Annu Rev Biophys. 2019 May 6;48:515-536. doi: 10.1146/annurev-biophys-052118-115308. Epub 2019 Apr 3. Annu Rev Biophys. 2019. PMID: 30943044 Review. - Dissecting the NUP107 complex: multiple components and even more functions.
González-Aguilera C, Askjaer P. González-Aguilera C, et al. Nucleus. 2012 Jul 1;3(4):340-8. doi: 10.4161/nucl.21135. Epub 2012 Jun 20. Nucleus. 2012. PMID: 22713280 Review.
Cited by
- Structure Determination of the Nuclear Pore Complex with Three-Dimensional Cryo electron Microscopy.
von Appen A, Beck M. von Appen A, et al. J Mol Biol. 2016 May 22;428(10 Pt A):2001-10. doi: 10.1016/j.jmb.2016.01.004. Epub 2016 Jan 12. J Mol Biol. 2016. PMID: 26791760 Free PMC article. Review. - Dissection of the NUP107 nuclear pore subcomplex reveals a novel interaction with spindle assembly checkpoint protein MAD1 in Caenorhabditis elegans.
Ródenas E, González-Aguilera C, Ayuso C, Askjaer P. Ródenas E, et al. Mol Biol Cell. 2012 Mar;23(5):930-44. doi: 10.1091/mbc.E11-11-0927. Epub 2012 Jan 11. Mol Biol Cell. 2012. PMID: 22238360 Free PMC article. - Structure of a trimeric nucleoporin complex reveals alternate oligomerization states.
Nagy V, Hsia KC, Debler EW, Kampmann M, Davenport AM, Blobel G, Hoelz A. Nagy V, et al. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17693-8. doi: 10.1073/pnas.0909373106. Epub 2009 Oct 1. Proc Natl Acad Sci U S A. 2009. PMID: 19805193 Free PMC article. - Molecular architecture of the inner ring scaffold of the human nuclear pore complex.
Kosinski J, Mosalaganti S, von Appen A, Teimer R, DiGuilio AL, Wan W, Bui KH, Hagen WJ, Briggs JA, Glavy JS, Hurt E, Beck M. Kosinski J, et al. Science. 2016 Apr 15;352(6283):363-5. doi: 10.1126/science.aaf0643. Science. 2016. PMID: 27081072 Free PMC article. - Evolutionary divergence of the nuclear pore complex from fungi to metazoans.
Chopra K, Bawaria S, Chauhan R. Chopra K, et al. Protein Sci. 2019 Mar;28(3):571-586. doi: 10.1002/pro.3558. Epub 2018 Dec 24. Protein Sci. 2019. PMID: 30488506 Free PMC article.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. Determining the architectures of macromolecular assemblies. Nature. 2007a;450:683–694. - PubMed
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007b;450:695–701. - PubMed
- Beck M, Lucic V, Forster F, Baumeister W, Medalia O. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature. 2007;449:611–615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous