Equine arteritis virus is delivered to an acidic compartment of host cells via clathrin-dependent endocytosis - PubMed (original) (raw)
Equine arteritis virus is delivered to an acidic compartment of host cells via clathrin-dependent endocytosis
Matthias Nitschke et al. Virology. 2008.
Abstract
Equine arteritis virus (EAV) is an enveloped, positive-stranded RNA virus belonging to the family Arteriviridae. Infection by EAV requires the release of the viral genome by fusion with the respective target membrane of the host cell. We have investigated the entry pathway of EAV into Baby Hamster Kidney cells (BHK). Infection of cells assessed by the plaque reduction assay was strongly inhibited by substances which interfere with clathrin-dependent endocytosis and by lysosomotropic compounds. Furthermore, infection of BHK cells was suppressed when clathrin-dependent endocytosis was inhibited by expression of antisense RNA of the clathrin-heavy chain before infection. These results strongly suggest that EAV is taken up via clathrin-dependent endocytosis and is delivered to acidic endosomal compartments.
Figures
Fig. 1
Role of clathrin-dependent endocytosis in infection of BHK cells by EAV. BHKasc cells were preincubated in the presence (tc+) and absence (tc−) of tetracycline. In the latter case antisense RNA against clathrin heavy chain is expressed suppressing clathrin-dependent endocytosis. A. Alexa Fluor 633 transferrin uptake. CHC expressing cells (tc+) display fluorescent transferrin (Tf) with high intensities in intracellular vesicles, whereas cells (tc−) with suppressed CHC expression show no or less intense intracellular internalisation of transferrin. Uptake was measured one min after addition of transferrin (37 °C). B.Infection of BHKasc cells by EAV. At 2h p.i. and 24h p.i. cells were fixed and examined, stained with a primary antibody against nucleocapsid protein and TRITC labelled secondary antibodies, and examined by confocal microscopy. Fluorescence images were recorded at identical gain settings. PC — phase contrast microscopy. DIC — differential interference microscopy.
Fig. 2
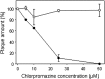
Influence of chlorpromazine on EAV infection of BHK-21 cells. BHK-21 cells were incubated with chlorpromazine and infection by EAV (filled circles) or by SV5 (open circles) was assessed by the plaque assay. Data present mean ± standard error of estimate of three independent experiments.
Fig. 3
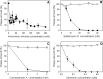
Influence of the lysosomotropic agents on EAV infection of BHK-21 cells. BHK-21 cells were incubated with ammonium chloride (squares) (A), with the vacuolar type H+-ATPase inhibitors (B) bafilomycin A1 (circles) and (C) concanamycin A (triangles up), and (D) with monensin (triangles down), and infection by EAV (filled symbols) or by SV5 (open symbols) was assessed by the plaque assay. Data present mean ± standard error of estimate of twelve (A) and at least three (B–D) and independent experiments.
Fig. 4
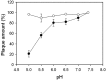
Influence of preincubation at acidic pH. EAV (filled circles) or SV5 (open circles) were incubated at the indicated pH for 1h at 37 °C. After pH neutralization of virus suspension, viruses were adsorbed to BHK cells and the plaque assay was performed (see Materials and methods). Data present mean ± standard error of estimate of three independent experiments.
Fig. 5
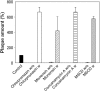
Low pH-bypass of inhibition of virus infection. BHK cells were pretreated as described above either with chlorpromazine (60 µM), concanamycin A (4nM), monensin (400nM), or MβCD. After the first 60 min of virus adsorption at 37 °C, the pH was lowered to 5.0 for 15 min. After neutralization, virus–cell complexes were incubated for further 45 min and the plaque assay was performed as described in Materials and methods. Control — mock pretreatment, no low pH exposure. w/o — without low pH exposure. w — with low pH exposure. Data present mean ± standard error of estimate of three independent experiments.
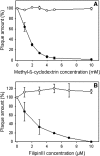
Fig. 6
Influence of cholesterol depletion on EAV infection of BHK-21 cells. (A) After pretreatment of BHK cells with MßCD or (B) incubation with filipin III infection of cells by EAV (filled circles) or by VSV (open circles) was assessed by the plaque assay. For treatment of the cells with substances see Materials and methods. Data present mean ± standard error of estimate of three independent experiments.
Similar articles
- Membrane proteins of arterivirus particles: structure, topology, processing and function.
Veit M, Matczuk AK, Sinhadri BC, Krause E, Thaa B. Veit M, et al. Virus Res. 2014 Dec 19;194:16-36. doi: 10.1016/j.virusres.2014.09.010. Epub 2014 Sep 30. Virus Res. 2014. PMID: 25278143 Free PMC article. Review. - Rab5 and Rab11 Are Required for Clathrin-Dependent Endocytosis of Japanese Encephalitis Virus in BHK-21 Cells.
Liu CC, Zhang YN, Li ZY, Hou JX, Zhou J, Kan L, Zhou B, Chen PY. Liu CC, et al. J Virol. 2017 Sep 12;91(19):e01113-17. doi: 10.1128/JVI.01113-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724764 Free PMC article. - Generation of a candidate live marker vaccine for equine arteritis virus by deletion of the major virus neutralization domain.
Castillo-Olivares J, Wieringa R, Bakonyi T, de Vries AA, Davis-Poynter NJ, Rottier PJ. Castillo-Olivares J, et al. J Virol. 2003 Aug;77(15):8470-80. doi: 10.1128/jvi.77.15.8470-8480.2003. J Virol. 2003. PMID: 12857916 Free PMC article. - Isolation and characterization of an arterivirus defective interfering RNA genome.
Molenkamp R, Rozier BC, Greve S, Spaan WJ, Snijder EJ. Molenkamp R, et al. J Virol. 2000 Apr;74(7):3156-65. doi: 10.1128/jvi.74.7.3156-3165.2000. J Virol. 2000. PMID: 10708432 Free PMC article. - Experiences with infectious cDNA clones of equine arteritis virus: lessons learned and insights gained.
Balasuriya UB, Zhang J, Go YY, MacLachlan NJ. Balasuriya UB, et al. Virology. 2014 Aug;462-463:388-403. doi: 10.1016/j.virol.2014.04.029. Epub 2014 Jun 7. Virology. 2014. PMID: 24913633 Free PMC article. Review.
Cited by
- Membrane proteins of arterivirus particles: structure, topology, processing and function.
Veit M, Matczuk AK, Sinhadri BC, Krause E, Thaa B. Veit M, et al. Virus Res. 2014 Dec 19;194:16-36. doi: 10.1016/j.virusres.2014.09.010. Epub 2014 Sep 30. Virus Res. 2014. PMID: 25278143 Free PMC article. Review. - Interactions of Equine Viruses with the Host Kinase Machinery and Implications for One Health and Human Disease.
Anderson C, Baha H, Boghdeh N, Barrera M, Alem F, Narayanan A. Anderson C, et al. Viruses. 2023 May 13;15(5):1163. doi: 10.3390/v15051163. Viruses. 2023. PMID: 37243249 Free PMC article. Review. - Co-translational processing of glycoprotein 3 from equine arteritis virus: N-glycosylation adjacent to the signal peptide prevents cleavage.
Matczuk AK, Kunec D, Veit M. Matczuk AK, et al. J Biol Chem. 2013 Dec 6;288(49):35396-405. doi: 10.1074/jbc.M113.505420. Epub 2013 Oct 18. J Biol Chem. 2013. PMID: 24142700 Free PMC article. - Genome-wide association study among four horse breeds identifies a common haplotype associated with in vitro CD3+ T cell susceptibility/resistance to equine arteritis virus infection.
Go YY, Bailey E, Cook DG, Coleman SJ, Macleod JN, Chen KC, Timoney PJ, Balasuriya UB. Go YY, et al. J Virol. 2011 Dec;85(24):13174-84. doi: 10.1128/JVI.06068-11. Epub 2011 Oct 12. J Virol. 2011. PMID: 21994447 Free PMC article. - Cell entry of arginine-rich peptides is independent of endocytosis.
Ter-Avetisyan G, Tünnemann G, Nowak D, Nitschke M, Herrmann A, Drab M, Cardoso MC. Ter-Avetisyan G, et al. J Biol Chem. 2009 Feb 6;284(6):3370-8. doi: 10.1074/jbc.M805550200. Epub 2008 Dec 1. J Biol Chem. 2009. PMID: 19047062 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources