Recent progress in tumor pH targeting nanotechnology - PubMed (original) (raw)
Review
Recent progress in tumor pH targeting nanotechnology
Eun Seong Lee et al. J Control Release. 2008.
Abstract
pH-sensitive polymeric micelles and nanogels have recently been developed to target slightly acidic extracellular pH environment of solid tumors. The pH targeting approach is regarded as a more general strategy than conventional specific tumor cell surface targeting approaches, because the acidic tumor microclimate is most common in solid tumors. When nanosystems are combined with triggered release mechanisms by endosomal or lysosomal acidity plus endosomolytic capability, the nanocarriers demonstrated to overcome multidrug resistance of various tumors. This review highlights recent progress of the pH-sensitive nanotechnology developed in Bae research group.
Figures
Figure 1
Mouse dorsal skin fold window chamber made of two symmetrical titanium frames. Tumor piece was inoculated into nu/nu mouse window chamber (A). After implanting MDA 213 breast cancer tumor piece, tumor blood vessels were growing in the window chamber Day 1 (B) and Day 15 (C). Normal blood vessel images after IV injection of DOX loaded pH-sensitive micelles (polyHis (Mw 5,000)-_b_-PEG (Mw 3,000)) 5 min and 60 min were shown in (D) and (E), respectively. The tumor blood vessels after i.v. injection of DOX loaded pH-insensitive and pH-sensitive micelles at the time course for 60 min were present in row (F) and row (G). The bright color is from DOX fluorescence. Reproduced with permission from reference [64].
Figure 2
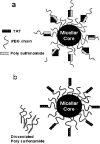
Schematic concept for a proposed drug delivery system: the carrier system consists of two components, poly(L-lactic acid)-_b_-PEG-TAT micelles and pH-sensitive poly(methacryloyl sulfadimethoxine-_b_-PEG. a) At normal blood pH, polysulfonamide is negatively charged, and when mixed with TAT, polysulfonamide shields the TAT by electrostatic interaction. Only PEG is exposed to the outside which could make the carrier long circulating; b) when the system experiences a decrease in pH (near tumor) polysulfonamide loses charge and detaches, exposing TAT for interaction with tumor cells. Reproduced with permission from reference [68].
Figure 3
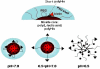
Schematic diagram depicting the central concept of pH induced biotin repositioning on the micelle. While above pH 7.0, biotin that is anchored on the micelle core via a pH-sensitive molecular chain actuator (polyHis) is shielded by PEG shell of the micelle; biotin is exposed on the micelle surface (6.5 < pH < 7.0) and can interact with cells, which facilitates biotin receptor-mediated endocytosis. When the pH is further lowered (pH < 6.5), the micelle destabilizes, resulting in enhanced drug release and disrupting cell membranes such as endosomal membrane. Reproduced with permission from reference [60].
Figure 4
pH-dependent cellular uptake of the micelles in cultured MCF-7 tumor cells caused by TAT exposure: pH 7.4 (•), pH 7.0 (■), pH 6.8 (▲). Particle size and size distributions of micelle suspensions (in PBS) at pH 7.4 were measured by dynamic light scattering. Each data point represents an average with standard deviation (n=3). Reproduced with permission from reference [45].
Figure 5
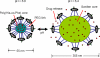
Schematic presentation of the virus-like nanogel. See the text for more details.
Figure 6
Release rate of DOX from DOX-loaded virus-like infectious nanogels. 150 μg of DOX was encapsulated into 1 mg of DOX-loaded VM-nanogels. The pH of the solution is stepwise adjusted to pH 7.4, pH 6.8 and pH 6.4 at one-hour intervals. Each data point represents an average with standard deviation (n=3). Reproduced with permission from reference [46].
Figure 7
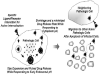
Virus-like nanogels infect cells selectively depending on specific interactions, kill the host cells, and migrate to neighboring cells as virus does to repeat the cycle.
Similar articles
- [Advances in the study of tumor pH-responsive polymeric micelles for cancer drug targeting delivery].
Xu JX, Tang JB, Zhao LH, Shen YQ. Xu JX, et al. Yao Xue Xue Bao. 2009 Dec;44(12):1328-35. Yao Xue Xue Bao. 2009. PMID: 21351464 Review. Chinese. - Fabrication and intracellular delivery of doxorubicin/carbonate apatite nanocomposites: effect on growth retardation of established colon tumor.
Hossain S, Yamamoto H, Chowdhury EH, Wu X, Hirose H, Haque A, Doki Y, Mori M, Akaike T. Hossain S, et al. PLoS One. 2013 Apr 16;8(4):e60428. doi: 10.1371/journal.pone.0060428. Print 2013. PLoS One. 2013. PMID: 23613726 Free PMC article. - pH-sensitive drug-delivery systems for tumor targeting.
He X, Li J, An S, Jiang C. He X, et al. Ther Deliv. 2013 Dec;4(12):1499-510. doi: 10.4155/tde.13.120. Ther Deliv. 2013. PMID: 24304248 Review. - [pH-sensitive polymeric micelles for the effective delivery of anti-cancer drug].
Na K. Na K. Korean J Gastroenterol. 2007 May;49(5):314-9. Korean J Gastroenterol. 2007. PMID: 17525519 Review. Korean.
Cited by
- pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery.
Wu H, Zhu L, Torchilin VP. Wu H, et al. Biomaterials. 2013 Jan;34(4):1213-22. doi: 10.1016/j.biomaterials.2012.08.072. Epub 2012 Oct 24. Biomaterials. 2013. PMID: 23102622 Free PMC article. - A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer.
Ai F, Ju Q, Zhang X, Chen X, Wang F, Zhu G. Ai F, et al. Sci Rep. 2015 Jun 2;5:10785. doi: 10.1038/srep10785. Sci Rep. 2015. PMID: 26035527 Free PMC article. - Engineering of Saposin C Protein Chimeras for Enhanced Cytotoxicity and Optimized Liposome Binding Capability.
Sandin SI, Gravano DM, Randolph CJ, Sharma M, de Alba E. Sandin SI, et al. Pharmaceutics. 2021 Apr 19;13(4):583. doi: 10.3390/pharmaceutics13040583. Pharmaceutics. 2021. PMID: 33921905 Free PMC article. - Acidic pH-Triggered Drug-Eluting Nanocomposites for Magnetic Resonance Imaging-Monitored Intra-arterial Drug Delivery to Hepatocellular Carcinoma.
Park W, Chen J, Cho S, Park SJ, Larson AC, Na K, Kim DH. Park W, et al. ACS Appl Mater Interfaces. 2016 May 25;8(20):12711-9. doi: 10.1021/acsami.6b03505. Epub 2016 May 16. ACS Appl Mater Interfaces. 2016. PMID: 27159350 Free PMC article. - Controlled release of doxorubicin from pH-responsive microgels.
Dadsetan M, Taylor KE, Yong C, Bajzer Z, Lu L, Yaszemski MJ. Dadsetan M, et al. Acta Biomater. 2013 Mar;9(3):5438-46. doi: 10.1016/j.actbio.2012.09.019. Epub 2012 Sep 25. Acta Biomater. 2013. PMID: 23022545 Free PMC article.
References
- Matei D. Novel agents in ovarian cancer. Expert Opin. Investig. Drugs. 2007;16:1227–1239. - PubMed
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007;608:1–22. - PubMed
- Cavaletti G, Bogliun G, Marzorati L, Zincone A, Marzola M, Colombo N, Tredici G. peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer. 1995;75:1141–1150. - PubMed
- Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–163. - PubMed
- Fojo T, Coley HM. The role of efflux pumps in drug-resistant metastatic breast cancer: new insights and treatment strategies. Clin. Breast Cancer. 2007;7:749–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA122356/CA/NCI NIH HHS/United States
- CA101850/CA/NCI NIH HHS/United States
- R01 CA122356-02/CA/NCI NIH HHS/United States
- R01 CA101850-04/CA/NCI NIH HHS/United States
- R01 CA101850/CA/NCI NIH HHS/United States
- CA122356/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources