Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase - PubMed (original) (raw)
Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase
Hyun Jung Park et al. Mol Cell Biol. 2008 Sep.
Abstract
The forkhead box M1 (FoxM1) transcription factor is overexpressed in many cancers, and in mouse models it is required for tumor progression. FoxM1 activates expression of the cell cycle genes required for both S and M phase progression. Here we demonstrate that FoxM1 is degraded in late mitosis and early G(1) phase by the anaphase-promoting complex/cyclosome (APC/C) E3 ubiquitin ligase. FoxM1 interacts with the APC/C complex and its adaptor, Cdh1. Expression of Cdh1 stimulated degradation of the FoxM1 protein, and depletion of Cdh1 resulted in stabilization of the FoxM1 protein in late mitosis and in early G(1) phase of the cell cycle. Cdh1 has been implicated in regulating S phase entry. We show that codepletion of FoxM1 inhibits early S phase entry observed in Cdh1-depleted cells. The N-terminal region of FoxM1 contains both destruction box (D box) and KEN box sequences that are required for targeting by Cdh1. Mutation of either the D box sequence or the KEN box sequence stabilized FoxM1 and blocked Cdh1-induced proteolysis. Cells expressing a nondegradable form of FoxM1 entered S phase rapidly following release from M phase arrest. Together, our observations show that FoxM1 is one of the targets of Cdh1 in late M or early G(1) phase and that its proteolysis is important for regulated entry into S phase.
Figures
FIG. 1.
Levels of FoxM1 protein decrease during late mitosis and early G1 phase of the cell cycle. (A) FoxM1 protein levels decrease in late mitosis and G1 phase of the cell cycle. HeLa cells were arrested at the G1-S boundary by double thymidine block, released into fresh medium, and harvested at the indicated times. Cell lysates were subjected to Western blot assays with antibodies specific to FoxM1, Aurora B kinase, Cdc20, and polo-like kinase 1 (Plk1) protein. β-Actin was used as a loading control. Cell cycle status at various time points following release from the double thymidine block was determined by flow cytometry. (B) FoxM1 is phosphorylated during the G2-M phase transition. Protein extracts were prepared from HeLa cells arrested in prometaphase by nocodazole treatment or in G1-S phase by double thymidine block. Extracts of nocodazole-arrested HeLa cells were treated with or without phosphatase and compared to double thymidine-arrested HeLa cell extracts. Migration of the FoxM1 protein in these extracts was determined by Western blotting with a FoxM1-specific antibody. (C) FoxM1 protein levels are reduced during late mitosis and G1 phase of the cell cycle. HeLa cells were released from double thymidine block (S phase) or nocodazole block (prometaphase) in the presence of cycloheximide (CHX) for the indicated times. To visualize FoxM1 protein levels, cell extracts were subjected to Western blotting with antibody specific to the FoxM1 protein.
FIG. 2.
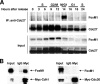
FoxM1 interacts with the APC/C-Cdh1 complex. (A) FoxM1 interacts with the Cdc27 subunit of the ubiquitin-APC/C complex during late mitosis and early G1 phase of the cell cycle. HeLa cells were synchronized at G1/S by double thymidine block, and protein extracts were prepared at the indicated hours after release from the double thymidine block. Cdc27 was immunoprecipitated with anti-Cdc27, and the presence of FoxM1 and Cdc27 proteins in the immunocomplexes was analyzed by Western blot assays with either the Cdc27 or FoxM1 antibody. (B) FoxM1 interacts with Cdh1 in co-IP assays. To determine the interaction between FoxM1 and the APC/C coactivator, i.e., Cdc20 or Cdh1, U2OS cells were transfected with T7 epitope-tagged FoxM1 (T7-FoxM1) and Myc epitope-tagged Cdh1 (Myc-Cdh1) or Cdc20 (Myc-Cdc20) expression vector. Cells were lysed after 24 h of transfection and subjected to co-IP assays using either antibody against the Myc epitope tag (anti-Myc) or control mouse immunoglobulin G, and immunoprecipitates were analyzed by Western blotting with antibodies specific to either FoxM1 or the Myc epitope tag.
FIG. 3.
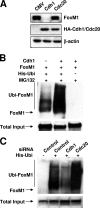
FoxM1 degradation is stimulated by Cdh1. (A) The FoxM1 protein is preferentially degraded by overexpression of the Cdh1 subunit of the ubiquitin-APC/C complex. U2OS cells were transfected with the T7-FoxM1 expression vector together with an expression vector for either hemagglutinin-Cdh1 (HA-Cdh1) or HA-Cdc20. The steady-state level of the FoxM1 protein was analyzed by Western blot assays with the FoxM1 antibody. (B) Cdh1-stimulated ubiquitination of the FoxM1 protein in vivo. U2OS cells were transfected with T7-FoxM1 along with Myc-Cdh1 expression vector or empty vector. Cells were lysed, and the lysates were bound to Ni-NTA beads. The bound fractions containing the ubiquitinated proteins were analyzed for polyubiquitination of the FoxM1 protein by Western blot assays with FoxM1 antibody. (C) Cdh1 is required for ubiquitination of endogenous FoxM1. U2OS cells were transfected with siRNA against Cdh1 or Cdc20 or with control siRNA. The cells were also transfected with a plasmid expressing His-ubiquitin (His-ubi). Lysates of the transfected cells were bound to Ni-NTA beads, and the bound fractions were analyzed with FoxM1 antibody in Western blot assays.
FIG. 4.
Depletion of Cdh1 increases FoxM1 levels. (A) Depletion of Cdh1 stabilizes FoxM1. HeLa cells were transfected with control siRNA or siRNA duplexes specific to Cdh1, Cdc20, or FoxM1. Seventy-two hours after siRNA transfection, levels of FoxM1 were analyzed by Western blot analysis with FoxM1 antibody. The levels of Cdh1 and Cdc20 were also analyzed by Western blotting, and β-actin was used as a loading control. FoxM1 mRNA levels were measured by quantitative reverse transcription-PCR as described in Materials and Methods. Data from a flow cytometric analysis of the siRNA-transfected cells are shown. (B) Depletion of Cdh1 in HeLa cells by siRNA transfection prevents reduction of FoxM1 protein levels in G1 phase of the cell cycle. The experimental scheme indicates the sequence of the double thymidine G1/S phase synchronization and Cdh1 siRNA transfection experiments with HeLa cells (upper panel). Cell extracts were prepared from the G1/S phase cells and from the cells at G1 phase. The extracts were subjected to Western blot analysis with FoxM1 antibody.
FIG. 5.
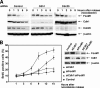
FoxM1 is a critical target of Cdh1 to inhibit premature S phase entry. (A) Diminished expression of Cdh1 in HeLa cells by siRNA transfection inhibits reduction of FoxM1 protein levels in late mitosis and G1 phase of the cell cycle. Control siRNA-, Cdc20 siRNA-, or Cdh1 siRNA-transfected HeLa cells were arrested in prometaphase by nocodazole, released, and then harvested at the indicated times following removal of nocodazole. Cell extracts were subjected to Western blot assays with anti-FoxM1, with β-actin as a loading control. (B) Codepletion of FoxM1 prevents aberrant S phase entry after Cdh1 depletion. HeLa cells were transfected with control siRNA, Cdh1 or FoxM1 siRNA, or a combination of FoxM1 siRNA and Cdh1 siRNA. After 72 h, cells were arrested in G2/M phase by nocodazole for 16 h and then released for the indicated times. BrdU (10 μM) was added to the culture medium 1 h before cells were harvested. BrdU-positive cells were determined by immunostaining as described in Materials and Methods. For each time point, the average percentage of BrdU-positive cells from three sets is plotted.
FIG. 6.
Codepletion of FoxM1 prevents premature S phase entry induced by Cdh1 depletion after serum induction. (A) FoxM1 levels are decreased in serum-starved cells. NIH 3T3 cells were serum starved for 24 h. For one set, during the last 6 h, MG132 was added to inhibit 26S proteasome-dependent proteolysis. (B) Depletion of Cdh1 prevents the decrease in FoxM1 protein levels in serum-depleted cells. Cdh1 siRNA was transfected into NIH 3T3 cells, and then the cells were serum starved for the indicated times. FoxM1 protein levels were determined by Western blot analysis. Data for flow cytometric analysis of the cells 0 h and 48 h after serum starvation are shown. (C) NIH 3T3 cells were transfected with the indicated siRNA. After 48 h of serum starvation, the siRNA-transfected cells were incubated with medium containing 10% fetal bovine serum for the indicated times. BrdU (10 μM) was added for 1 h before the cells were harvested. BrdU-positive cells were stained with anti-BrdU antibody and counted as described in Materials and Methods. For each point, the average percentage of BrdU-positive cells from three sets is plotted.
FIG. 7.
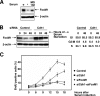
FoxM1 degradation requires retention of both the D box and KEN box sequences. (A) Schematic representation of the FoxM1 protein depicting two D box sequences, a KEN box sequence, and the amino acid positions of these sequences. Also indicated on the FoxM1 protein are the winged-helix DNA binding domain (DBD), the C-terminal transcriptional activation domain (TAD), and the amino acid positions of these domains. (B) Deletion of the D box sequence or mutation in the KEN box of the FoxM1 protein prevents degradation by Cdh1. U2OS cells were transfected with either a WT Myc-FoxM1, Myc-FoxM1 D box deletion mutant (ΔD), or Myc-FoxM1 KEN box mutant (KEN) expression vector, with or without Myc-Cdh1 expression plasmid, and protein extracts were evaluated for the FoxM1 protein level by Western blot analysis with FoxM1 antibody. (C) The ΔD/KEN double mutant protein exhibits enhanced stability in G1 phase. Cells expressing WT or mutant FoxM1 were synchronized by nocodazole and then released for the indicated times in the presence of cycloheximide (100 μg/ml). The extracts were evaluated for FoxM1 levels by Western blot assays with the FoxM1 antibody. (D) Cells expressing a nondegradable mutant (ΔD/KEN) enter S phase earlier than do WT FoxM1-expressing cells. Cells expressing the WT or the nondegradable form (ΔD/KEN) of the FoxM1 protein were arrested at prometaphase with nocodazole and then released for the indicated times. BrdU (10 μM) was added for 1 hour before fixation and staining. BrdU-positive cells were analyzed by immunostaining with anti-BrdU and anti-mouse-fluorescein isothiocyanate antibodies, as described in Materials and Methods. (E) Cdh1 depletion further accelerates S phase entry of cells expressing the nondegradable form of FoxM1. Cells expressing the nondegradable form (ΔD/KEN) of FoxM1 or the parental line were transfected with control or Cdh1 siRNA. After 72 h of transfection, cells were arrested at G2/M phase by nocodazole treatment for 16 h and then released into fresh medium for the indicated times. One hour prior to fixation, cells were incubated with BrdU as described for panel D. For each point, the average percentage of BrdU-positive cells from three sets is plotted.
References
- Bashir, T., N. V. Dorrello, V. Amador, D. Guardavaccaro, and M. Pagano. 2004. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428190-193. - PubMed
- Carlsson, P., and M. Mahlapuu. 2002. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 2501-23. - PubMed
- Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1193-199. - PubMed
- Costa, R. H., V. V. Kalinichenko, A. X. Holterman, and X. Wang. 2003. Transcription factors in liver development, differentiation, and regeneration. Hepatology 381331-1347. - PubMed
- Douard, R., S. Moutereau, P. Pernet, M. Chimingqi, Y. Allory, P. Manivet, M. Conti, M. Vaubourdolle, P. H. Cugnenc, and S. Loric. 2006. Sonic Hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery 139665-670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA 124488/CA/NCI NIH HHS/United States
- R01 CA124488/CA/NCI NIH HHS/United States
- R01 AG021842/AG/NIA NIH HHS/United States
- DK 44525/DK/NIDDK NIH HHS/United States
- R01 DK054687/DK/NIDDK NIH HHS/United States
- AG021842/AG/NIA NIH HHS/United States
- R01 DK044525/DK/NIDDK NIH HHS/United States
- DK068503/DK/NIDDK NIH HHS/United States
- R01 CA100035/CA/NCI NIH HHS/United States
- CA 100035/CA/NCI NIH HHS/United States
- R01 DK068503/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous