Foxp3+ regulatory T cells maintain immune homeostasis in the skin - PubMed (original) (raw)
Foxp3+ regulatory T cells maintain immune homeostasis in the skin
Jan C Dudda et al. J Exp Med. 2008.
Abstract
Cutaneous immune responses must be tightly controlled to prevent unwanted inflammation in response to innocuous antigens, while maintaining the ability to combat skin-tropic pathogens. Foxp3(+) regulatory T (T reg) cells are potent immune regulators and are found at high frequency in both human and mouse skin. Although T reg cells migrate to the skin and can dampen immune responses during experimentally induced inflammation or infection, the importance of cutaneous T reg cells for maintaining normal immune homeostasis in the skin has not been addressed. To selectively block T reg cell function in the skin, we restored the T reg cell compartment in Foxp3-deficient scurfy mice with cells whose ability to migrate to the skin was impaired because of targeted mutation of alpha-1,3-fucosyltransferase VII (Fut7). Although Fut7-deficient T reg cells were present at normal frequency and could function in all other tissues examined, these animals rapidly developed severe cutaneous inflammation. Thus, skin-resident T reg cell are essential for maintaining normal immune homeostasis at this site.
Figures
Figure 1.
T reg cells transferred into sf mice up-regulate cutaneous homing receptors and accumulate in the skin. (A) Homing receptor expression in LNs and spleen 4 wk after transfer of T reg cells into a neonatal sf mouse was compared with that in an 8-wk-old Foxp3gfp mouse by flow cytometry. Numbers indicate the frequency of positive cells among the Foxp3+ T reg cells. Plots are gated on total live cells (top left) or CD4+Foxp3+ cells (all other graphs). (B) Flow cytometry analysis of CD103 and Foxp3 expression by gated CD4+ T cells isolated from the indicated tissues 4 wk after T reg cell transfer into neonatal sf mice. Data are representative of three independent experiments.
Figure 2.
Phenotypic and functional characterization of FuT7−/−-T reg cells. (A) Frequency of Foxp3+ T reg cells among CD4+ T cells in peripheral LNs and spleens of 8-wk-old WT (shaded bars) or FuT7−/− (open bars) mice. (B) Frequency of CD4+Foxp3+ T reg cells expressing the indicated homing receptors in peripheral LNs (PLN) or spleens of WT and FuT7−/− mice. Data in A and B are the mean and SD of values from three age-matched mice/group. Significance was measured using two-tailed, unpaired Student's t tests. *** indicates P < 0.0001; P > 0.05 for all other comparisons. (C) Proliferation of CD4+CD25− effector T cells as measured by CFSE dilution after 96 h of culture with CD4-depleted splenocytes in the presence or absence of anti-CD3 and -CD28 antibodies (top) and the indicated ratio of WT- or FuT7−/−-T reg cells (middle and bottom). Representative results of one out of two independent experiments.
Figure 3.
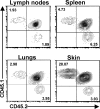
Accumulation of FuT7−/−-T reg cells in the skin is selectively impaired. WT- (CD45.1+) and FuT7−/−- (CD45.2+) T reg cells were transferred into 2-d-old sf mice (CD45.1+CD45.2+), and tissue distribution was analyzed 6 wk later by flow cytometry analysis of CD45.1 and CD45.2 expression by gated CD4+ T cells isolated from the indicated tissues. Representative results of one out of three independent experiments.
Figure 4.
Blocking T reg cell migration to the skin results in cutaneous inflammation. (A) Photomicrographs of hematoxylin and eosin–stained sections from skin, liver, and lung of sf mice left untreated (top; 21 d old) or given WT- (middle; 53 d old) or FuT7−/−- (bottom; 35 d old) T reg cells. Bars, 100 μm. (B) Flow cytometry analysis of CD44 and CD45RB expression by gated CD4+Foxp3− cells from pooled LNs and spleens of age-matched WT and sf mice (top; 21 d old) or sf mice given WT- or FuT7−/−-T reg cells (bottom; 35 d old). Numbers indicate the frequency of activated CD44high/CD45RBlow cells. (C) Flow cytometry analysis of CD45.1 and CD45.2 expression by gated CD4+ T cells isolated from the indicated tissues of CD45.1+sf mice given CD45.2+ WT- or FuT7−/−-T reg cells 5 wk before. Percentages indicate the frequency of CD45.2+ T reg cells among CD4+ cells. Data are representative of three to six experiments.
Similar articles
- Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma.
Klein S, Kretz CC, Ruland V, Stumpf C, Haust M, Hartschuh W, Hartmann M, Enk A, Suri-Payer E, Oberle N, Krammer PH, Kuhn A. Klein S, et al. Ann Rheum Dis. 2011 Aug;70(8):1475-81. doi: 10.1136/ard.2009.116525. Epub 2010 Nov 19. Ann Rheum Dis. 2011. PMID: 21097800 - Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease.
Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Sather BD, et al. J Exp Med. 2007 Jun 11;204(6):1335-47. doi: 10.1084/jem.20070081. Epub 2007 Jun 4. J Exp Med. 2007. PMID: 17548521 Free PMC article. - Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression.
Peterson RA. Peterson RA. Toxicol Pathol. 2012;40(2):186-204. doi: 10.1177/0192623311430693. Epub 2012 Jan 5. Toxicol Pathol. 2012. PMID: 22222887 Review. - Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease.
Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Lahl K, et al. J Exp Med. 2007 Jan 22;204(1):57-63. doi: 10.1084/jem.20061852. Epub 2007 Jan 2. J Exp Med. 2007. PMID: 17200412 Free PMC article. - Phenotypical and functional specialization of FOXP3+ regulatory T cells.
Campbell DJ, Koch MA. Campbell DJ, et al. Nat Rev Immunol. 2011 Feb;11(2):119-30. doi: 10.1038/nri2916. Nat Rev Immunol. 2011. PMID: 21267013 Free PMC article. Review.
Cited by
- Immunogenicity and tolerance induction in vascularized composite allotransplantation.
Sun JA, Adil A, Biniazan F, Haykal S. Sun JA, et al. Front Transplant. 2024 Feb 13;3:1350546. doi: 10.3389/frtra.2024.1350546. eCollection 2024. Front Transplant. 2024. PMID: 38993748 Free PMC article. Review. - Regulatory T cells in skin mediate immune privilege of the hair follicle stem cell niche.
Cohen JN, Gouirand V, Macon CE, Lowe MM, Boothby IC, Moreau JM, Gratz IK, Stoecklinger A, Weaver CT, Sharpe AH, Ricardo-Gonzalez RR, Rosenblum MD. Cohen JN, et al. Sci Immunol. 2024 Jan 5;9(91):eadh0152. doi: 10.1126/sciimmunol.adh0152. Epub 2024 Jan 5. Sci Immunol. 2024. PMID: 38181095 Free PMC article. - Intragraft regulatory T cells in the modern era: what can high-dimensional methods tell us about pathways to allograft acceptance?
Bei KF, Moshkelgosha S, Liu BJ, Juvet S. Bei KF, et al. Front Immunol. 2023 Nov 23;14:1291649. doi: 10.3389/fimmu.2023.1291649. eCollection 2023. Front Immunol. 2023. PMID: 38077395 Free PMC article. Review. - Regulatory T cells in skin regeneration and wound healing.
Knoedler S, Knoedler L, Kauke-Navarro M, Rinkevich Y, Hundeshagen G, Harhaus L, Kneser U, Pomahac B, Orgill DP, Panayi AC. Knoedler S, et al. Mil Med Res. 2023 Oct 23;10(1):49. doi: 10.1186/s40779-023-00484-6. Mil Med Res. 2023. PMID: 37867188 Free PMC article. Review. - Obesity-induced dysregulation of skin-resident PPARγ+ Treg cells promotes IL-17A-mediated psoriatic inflammation.
Sivasami P, Elkins C, Diaz-Saldana PP, Goss K, Peng A, Hamersky M 4th, Bae J, Xu M, Pollack BP, Horwitz EM, Scharer CD, Seldin L, Li C. Sivasami P, et al. Immunity. 2023 Aug 8;56(8):1844-1861.e6. doi: 10.1016/j.immuni.2023.06.021. Epub 2023 Jul 20. Immunity. 2023. PMID: 37478855 Free PMC article.
References
- Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. - PubMed
- Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. - PubMed
- Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. - PubMed
- Kim, J.M., J.P. Rasmussen, and A.Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197. - PubMed
- Powrie, F., M.W. Leach, S. Mauze, L.B. Caddle, and R.L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461–1471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK072295/DK/NIDDK NIH HHS/United States
- DK072295/DK/NIDDK NIH HHS/United States
- AI067750/AI/NIAID NIH HHS/United States
- R56 AI067750/AI/NIAID NIH HHS/United States
- R21 AI069889/AI/NIAID NIH HHS/United States
- R01 AI067750/AI/NIAID NIH HHS/United States
- AI069889/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases