Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain - PubMed (original) (raw)
Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain
Iwona Sadzak et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The transcription factor Stat1 plays an essential role in responses to interferons (IFNs). Activation of Stat1 is achieved by phosphorylation on Y701 that is followed by nuclear accumulation. For full transcriptional activity and biological function Stat1 must also be phosphorylated on S727. The molecular mechanisms underlying the IFN-induced S727 phosphorylation are incompletely understood. Here, we show that both Stat1 Y701 phosphorylation and nuclear translocation are required for IFN-induced S727 phosphorylation. We further show that Stat1 mutants lacking the ability to stably associate with chromatin are poorly serine-phosphorylated in response to IFN-gamma. The S727 phosphorylation of these mutants is restored on IFN-beta treatment that induces the formation of the ISGF3 complex (Stat1/Stat2/Irf9) where Irf9 represents the main DNA binding subunit. These findings indicate that Stat1 needs to be assembled into chromatin-associated transcriptional complexes to become S727-phosphorylated and fully biologically active in response to IFNs. This control mechanism, which may be used by other Stat proteins as well, restricts the final activation step to the chromatin-tethered transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
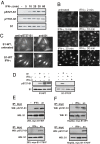
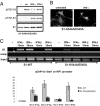
Y701 is required for S727 phosphorylation induced by type I or type II IFNs. (A) S1-WT cells were treated with IFN-γ for indicated times and phosphorylation of S727 was examined by Western blot analysis of whole-cell extracts with pS727-S1 antibody. The membrane was reprobed with antibody to phosphorylated Y701 (pY701-S1) and Stat1 (S1). (B) Same cells as in A were stimulated with IFN-γ for indicated times and localization of Stat1 was examined by immunofluorescence by using a Stat1 antibody. (C) Localization of S727-phosphorylated Stat1 was examined in untreated or IFN-γ-treated S1-WT cells by double immunofluorescence by using antibodies to pS727-S1 and total Stat1 (S1). (D) S1-WT and S1-Y701F cells were treated for 30 min with anisomycin (an), IFN-γ, IFN-β, or left untreated. Western blot analysis of whole-cell extracts was performed with pS727-S1 antibody, or with Stat1 antibody for loading control. (E) 293HEK cells stably expressing myc-tagged Stat1-WT (293, myc-S1-WT; Upper) or Stat1-Y701F (293, myc-S1-Y701F; Lower) were treated for 30 min with IFN-γ or anisomycin (an). Myc-tagged proteins were immunoprecipitated from extracts by using myc antibody, and analyzed by Western blot analysis with pS727-S1, or S1 (loading control) antibodies. (F) HepG2 cells stably expressing myc-tagged Stat1-WT (HepG2, myc-S1-WT; Upper) or Stat1-Y701F (HepG2, myc-S1-Y701F; Lower) were treated and analyzed as in E.
Fig. 2.
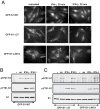
Nuclear import is required for IFN-induced S727 phosphorylation of Stat1. (A) Cells stably expressing GFP-S1-WT, GFP-S1-Δ27, or GFP-S1-L407 constructs were treated for 30 min with IFN-γ, IFN-β, or left untreated. Localization of Stat1 was monitored by GFP fluorescence. GFP-S1-WT (B) or GFP-S1-L407A and GFP-S1-Δ27 (C) cells were treated for 30 min with anisomycin (an), IFN-γ, or IFN-β. Phosphorylation of immunoprecipitated Stat1 was detected by Western blot analysis by using pS727-S1 and pY701-S1 antibodies. Membrane was reprobed with Stat1 antibody (S1).
Fig. 3.
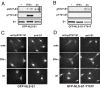
Nuclear Stat1 is not phosphorylated on S727 unless it becomes Y701-phosphorylated in response to IFN-γ. (A and B) Stat1 was immunoprecipitated from extracts of GFP-NLS-S1 or GFP-NLS-S1-Y701F cells, respectively, treated for 30 min with IFN-γ or anisomycin (an). Phosphorylation of Stat1 on S727 and Y701 was assayed by Western blot analysis by using antibodies to phosphorylated S727 (pS727-S1) and Y701 (pY701-S1). The membrane was reprobed with Stat1 antibody (S1). (C and D) Localization of GFP-NLS-S1 or GFP-NLS-S1-Y701F (respectively) and their S727-phosphorylated forms was investigated by immunofluorescence microscopy by using double staining with antibodies to pS727-S1 and total Stat1 (S1). Cells were treated with IFN-γ or anisomycin (an).
Fig. 4.
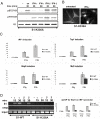
Stat1 deficient in DNA binding displays strongly reduced S727-phosphorylation in response to IFN-γ. (A) Stat1 was immunoprecipitated from extracts of S1-K336A cells treated with anisomycin (an), IFN-γ, or IFN-β for times indicated. Phosphorylation of Stat1 was assayed by Western blot analysis by using antibodies to phosphorylated S727 (pS727-S1) and Y701 (pY701-S1). The membrane was reprobed with Stat1 antibody (S1). (B) Localization of the S1-K336A mutant in cells treated for 30 min with IFN-γ as revealed by immunofluorescence using S1 antibody. (C) S1-WT and S1-K336A cells were treated with IFN-γ or IFN-β for 1 h (for Irf1 and Mx2) or 4 h (for Tap1 and Gbp2), and total RNA was isolated for qRT-PCR. The graphs show induction of mRNA of Irf1, Tap1, Ggb2, and Mx2. (D) S1-K336A mutant is not recruited to the Irf1 GAS on treatment with IFN-γ or IFN-β (for 15 or 30 min) as revealed by ChIP (Upper) or qChIP (Lower).
Fig. 5.
Stable association of Stat1 with chromatin is required for efficient IFN-induced S727 phosphorylation. (A) Stat1 was immunoprecipitated from extracts of S1-K544A/E545A cells treated for 30 min with anisomycin (an), IFN-γ, or IFN-β. Phosphorylation of Stat1 was assayed by Western blot analysis by using antibodies to phosphorylated S727 (pS727-S1) and Y701 (pY701-S1). The membrane was reprobed with Stat1 antibody (S1). (B) Localization of the S1-K544A/E545A linker domain mutant in cells treated for 30 min with IFN-γ as revealed by immunofluorescence by using S1 antibody. (C) Recruitment of the S1- K544A/E545A mutant to the Irf1 GAS is strongly reduced compared with S1-WT in cells treated with IFN-γ or IFN-β (for 15 or 30 min) as revealed by ChIP (Upper) or qChIP (Lower).
Similar articles
- Distinct modes of action applied by transcription factors STAT1 and IRF1 to initiate transcription of the IFN-gamma-inducible gbp2 gene.
Ramsauer K, Farlik M, Zupkovitz G, Seiser C, Kröger A, Hauser H, Decker T. Ramsauer K, et al. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2849-54. doi: 10.1073/pnas.0610944104. Epub 2007 Feb 9. Proc Natl Acad Sci U S A. 2007. PMID: 17293456 Free PMC article. - Adenosine blocks IFN-gamma-induced phosphorylation of STAT1 on serine 727 to reduce macrophage activation.
Barnholt KE, Kota RS, Aung HH, Rutledge JC. Barnholt KE, et al. J Immunol. 2009 Nov 15;183(10):6767-77. doi: 10.4049/jimmunol.0900331. Epub 2009 Oct 21. J Immunol. 2009. PMID: 19846878 Free PMC article. - Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-gamma.
Nair JS, DaFonseca CJ, Tjernberg A, Sun W, Darnell JE Jr, Chait BT, Zhang JJ. Nair JS, et al. Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):5971-6. doi: 10.1073/pnas.052159099. Epub 2002 Apr 23. Proc Natl Acad Sci U S A. 2002. PMID: 11972023 Free PMC article. - A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.
Michalska A, Blaszczyk K, Wesoly J, Bluyssen HAR. Michalska A, et al. Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review. - The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.
Blaszczyk K, Nowicka H, Kostyrko K, Antonczyk A, Wesoly J, Bluyssen HA. Blaszczyk K, et al. Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
Cited by
- Astrocyte-induced Cdk5 expedites breast cancer brain metastasis by suppressing MHC-I expression to evade immune recognition.
Yuzhalin AE, Lowery FJ, Saito Y, Yuan X, Yao J, Duan Y, Ding J, Acharya S, Zhang C, Fajardo A, Chen HN, Wei Y, Sun Y, Zhang L, Xiao Y, Li P, Lorenzi PL, Huse JT, Fan H, Zhao Z, Hung MC, Yu D. Yuzhalin AE, et al. Nat Cell Biol. 2024 Oct;26(10):1773-1789. doi: 10.1038/s41556-024-01509-5. Epub 2024 Sep 20. Nat Cell Biol. 2024. PMID: 39304713 - Enhancing immunotherapy outcomes by targeted remodeling of the tumor microenvironment via combined cGAS-STING pathway strategies.
Huang M, Cha Z, Liu R, Lin M, Gafoor NA, Kong T, Ge F, Chen W. Huang M, et al. Front Immunol. 2024 May 16;15:1399926. doi: 10.3389/fimmu.2024.1399926. eCollection 2024. Front Immunol. 2024. PMID: 38817608 Free PMC article. Review. - PM2.5 Extracts Induce INFγ-Independent Activation of CIITA, MHCII, and Increases Inflammation in Human Bronchial Epithelium.
Jirau-Colón H, Jiménez-Vélez BD. Jirau-Colón H, et al. Toxics. 2024 Apr 16;12(4):292. doi: 10.3390/toxics12040292. Toxics. 2024. PMID: 38668515 Free PMC article. - Unveiling the impact of CDK8 on tumor progression: mechanisms and therapeutic strategies.
Yin X, He Z, Chen K, Ouyang K, Yang C, Li J, Tang H, Cai M. Yin X, et al. Front Pharmacol. 2024 Mar 28;15:1386929. doi: 10.3389/fphar.2024.1386929. eCollection 2024. Front Pharmacol. 2024. PMID: 38606172 Free PMC article. Review. - Reciprocal inhibition between TP63 and STAT1 regulates anti-tumor immune response through interferon-γ signaling in squamous cancer.
Jiang Y, Zheng Y, Zhang YW, Kong S, Dong J, Wang F, Ziman B, Gery S, Hao JJ, Zhou D, Zhou J, Ho AS, Sinha UK, Chen J, Zhang S, Yin C, Wei DD, Hazawa M, Pan H, Lu Z, Wei WQ, Wang MR, Koeffler HP, Lin DC, Jiang YY. Jiang Y, et al. Nat Commun. 2024 Mar 20;15(1):2484. doi: 10.1038/s41467-024-46785-9. Nat Commun. 2024. PMID: 38509096 Free PMC article.
References
- Levy DE, Darnell JE., Jr Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. - PubMed
- Varinou L, et al. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity. 2003;19:793–802. - PubMed
- Stancato LF, David M, Carter-Su C, Larner AC, Pratt WB. Preassociation of STAT1 with STAT2 and STAT3 in separate signalling complexes prior to cytokine stimulation. J Biol Chem. 1996;271:4134–4137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous