Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies - PubMed (original) (raw)
Meta-Analysis
Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies
Hedy Kober et al. Neuroimage. 2008.
Abstract
We performed an updated quantitative meta-analysis of 162 neuroimaging studies of emotion using a novel multi-level kernel-based approach, focusing on locating brain regions consistently activated in emotional tasks and their functional organization into distributed functional groups, independent of semantically defined emotion category labels (e.g., "anger," "fear"). Such brain-based analyses are critical if our ways of labeling emotions are to be evaluated and revised based on consistency with brain data. Consistent activations were limited to specific cortical sub-regions, including multiple functional areas within medial, orbital, and inferior lateral frontal cortices. Consistent with a wealth of animal literature, multiple subcortical activations were identified, including amygdala, ventral striatum, thalamus, hypothalamus, and periaqueductal gray. We used multivariate parcellation and clustering techniques to identify groups of co-activated brain regions across studies. These analyses identified six distributed functional groups, including medial and lateral frontal groups, two posterior cortical groups, and paralimbic and core limbic/brainstem groups. These functional groups provide information on potential organization of brain regions into large-scale networks. Specific follow-up analyses focused on amygdala, periaqueductal gray (PAG), and hypothalamic (Hy) activations, and identified frontal cortical areas co-activated with these core limbic structures. While multiple areas of frontal cortex co-activated with amygdala sub-regions, a specific region of dorsomedial prefrontal cortex (dmPFC, Brodmann's Area 9/32) was the only area co-activated with both PAG and Hy. Subsequent mediation analyses were consistent with a pathway from dmPFC through PAG to Hy. These results suggest that medial frontal areas are more closely associated with core limbic activation than their lateral counterparts, and that dmPFC may play a particularly important role in the cognitive generation of emotional states.
Figures
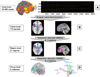
Fig. 1
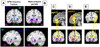
Schematic representation of the procedures for multilevel kernel density analysis (MKDA). (A) Peak coordinates in three of the 437 comparison maps included in this meta-analysis. (B) Peak coordinates in each map were separately convolved with a 10 mm kernel, generating comparison indicator maps (CIMs) of values 0 or 1 (1 shown in black). (C) The weighted average of the CIMs (weights based on sample size and analysis type) is thresholded by the maximum proportion of activated comparison maps expected under the null hypothesis (shown in D) to produce significant results. (E) Significant results: yellow voxels are familywise error rate (FWER) corrected at p<.05. Other colored regions are FWER corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01(pink).
Fig. 2
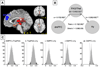
Schematic representation of the procedures for multivariate co-activation analysis. (A) Indicator matrix of comparison indicator map (CIM) values for suprathreshold voxels used as input — those that were found to be consistently and significantly activated in the previous MKDA analysis. Rows are contrasts, and columns are significant voxels. (B) Singular value decomposition was applied to these voxels, resulting in 172 parcels, or functional subregion of contiguous suprathreshold voxels that have similar patterns of activation across studies. (C) Parcels were subjected to dimension reduction (Nonmetric Multidimensional Scaling; NMDS) and clustering, resulting in 21 regions that were consistently co-activated across emotion studies. (D) The procedure described in (C) was repeated a second time, resulting in a best-estimate of 6 functional groups, shown in schematic view only (see Fig. 7 for high-resolution image of the functional groups). The groups describe functional relationships among large-scale anatomical regions.
Fig. 3
Plots showing results of the NMDS and clustering process. (A) Plot of observed dissimilarities between the parcels (in the process of clustering parcels into regions), against the model-implied distance. A non-linear relationship indicates that the metric model is inadequate. The nonlinear relationship suggests that NMDS is a more appropriate procedure than linear methods in this case. (B) _Z_-scores of clustering quality relative to the clustering quality of permuted data (_y_-axis) plotted as a function of number of clusters in the solution (_x_-axis). A 21-cluster solution was associated with the greatest improvement over permuted data, and was therefore chosen as the best estimate. (C) Cluster quality for observed data (vertical line) compared with a histogram of clustering quality for permuted data for the 21-cluster solution. Figs. 3D–F show similar plots for the second iteration of the algorithm, clustering regions into large-scale groups. (D) NMDS is indicated by the non-linear relationship. (E) A six-cluster solution was associated with the highest improvement over permuted data, and was therefore chosen. (F) The quality of the selected six-cluster solution (compared with permuted solutions) is indicated by the vertical line.
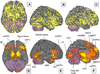
Fig. 4
(A–C) Un-weighted peak activations from all 437 contrasts in our meta-analysis are plotted on the lateral, orbital, and medial surfaces on the brain, respectively. Activations across studies are distributed throughout the cortex, though clusters of consistent results are concentrated in some areas. (D×F) Regions that were consistently activated across neuroimaging studies as determined by multi-level kernel density analysis. To achieve significance in our analysis, any single voxel had to be activated by at least ∼4% of the contrasts in our meta-analysis (e.g. 18 contrasts or more, depending on the study weights). Yellow voxels are family-wise error rate (FWER) corrected at p<.05. Other colored regions are Family-wise Error Rate corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01(pink). See Table 1 for abbreviations of brain region names.
Fig. 5
(A) Unweighted peak activations from all the 437 contrasts in our meta-analysis are plotted on the subcortical surface. (B) Regions that were consistently activated across neuroimaging studies as determined by the meta-analysis are plotted on the subcortical surface. (C) Significant activations in the Cerebellum (CB). (D–F) Cortical as well as subcortical regions of activation are shown in sagittal, coronal, and axial slices. Yellow voxels are family-wise error rate (FWER) corrected at p<.05. Other colored regions are FWER corrected for spatial extent at p<.05 with primary alpha levels of .001 (orange), and .01(pink). See Table 1 for abbreviations.
Fig. 6
Detailed maps of orbitofrontal and visuotopic subregions. (A) Orbitofrontal and visuotopic subregions as well as significant activations are overlaid on the orbital surface. Orbitofrontal regions are as described by Ongur, Ferry, and Price (2003) as implemented in Caret software (v5.5;
http://brainmap.wustl.edu/caret/
). (B) An enlarged view of the orbitofrontal cortex. Consistent activations are centered Area 47 m/L, ventral anterior insula, and frontal operculum bilaterally. (C) An enlarged ventral view of visuotopic regions, as defined in Caret (D. C. Van Essen, 2004). Activations are centered in V1, ventral V2, V8, and MT+, with extension into V4v and VP. (D) A flat map of the cortical surface of the right hemisphere, overlaid by both orbitofrontal and visuotopic regions, and significant activations. (E) A flat map of the cortical surface of the left hemisphere, overlaid by both orbitofrontal and visuotopic regions, and significant activations. See Table 1 for abbreviations.
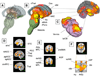
Fig. 7
(A–F) The six functional groups revealed by our multivariate analysis are depicted in 3D rendering on the single-subject brain. Regions in each group are rendered in a unique color. (G) To visualize the relationships among the regions in each group, both regions and co-activation lines are displayed on a “flattened” map of the connectivity space along the first two dimensions determined by NMDS (see Methods). Colors correspond to those in panels A–F and identify each network. Points closer together on the graph tend to have stronger positive co-activation, and connected lines represent significant Tau-b (τ) association values between pairs of regions. The connectivity map has been “pruned” such that the relationships depicted are direct, meaning that they were not completely mediated by any other single intervening region. Direct relationships were assessed by mediation analyses considering each possible mediating region in turn, with 1000 bootstrap samples per analysis. See Table 1 for abbreviations.
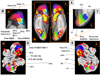
Fig. 8
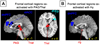
(A) Amygdala sub-regions specified in the SPM Anatomical Toolbox. The Basolateral Amygdala (BL) is depicted in magenta; the Centro-Medial complex (CM) is depicted in blue; the Superficial Amygdala (SF) is depicted in cyan. (B) Amygdala sub-regions identified in this meta-analysis. The Basolateral Amygdala is depicted in magenta (BL); the Superior Amygdala (SA) encompasses both the Superficial and CentroMedial complex, and is depicted in cyan. (C–E) Co-activation of frontal and amygdala sub-regions. Areas that were co-activated with three or more amygdala regions are depicted yellow; dmPFC, the only region co-activated with only one amygdala region, is depicted in orange.
Fig. 9
Co-activation of frontal and subcortical regions. (A) Frontal cortical regions that are co-activated with PAG. Color of activated areas indicate the network to which they belong — dark blue for the mPFC network (dmPFC, rdACC), light blue for the Cognitive/Motor network (rFrOP), and red for the Core Limbic network (PAG/Thal, the region tested for co-activity with frontal regions). (B) Frontal cortical regions that are co-activated with Hy (shown in red). Colors are as in (A). See Table 1 for abbreviations.
Fig. 10
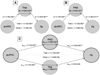
Mediation model and analyses for the association between dmPFC, Hy, and PAG/Thal. (A) Visualization of the locations of regions tested in the model and their connectivity in the model tested. (B) PAG/Thal is a complete mediator of dmPFC–Hy co-activation. Path coefficients are shown next to arrows indicating each link in the analysis, with standard errors in parentheses. a refers to the path from dmPFC to PAG/Thal. b refers to the direct link between PAG/Thal and Hy. c refers to the total association between dmPFC and Hy, without the mediator (PAG/Thal). *p<.05; **, p<.01; ***, p<.001, two-tailed. (C) Bootstrap distributions for this mediation analysis, based on 10,000 bootstrap samples. The histogram shows the bootstrapped sampling distribution, with the tails (p<.05) shaded in dark gray. The vertical line indicates the null hypothesis path coefficient value (zero). A significant result occurs when the null hypothesis value falls in the tail of the bootstrapped sampling distribution. See Table 1 for abbreviations.
Fig. 11
Thal and PAG as separate mediators of the dmPFC–Hy co-activation. Abbreviations for path coefficients are as in Fig. 9. (A) Thal is a complete mediator of the dmPFC–Hy co-activation. (B) PAG is a complete mediator of the dmPFC–Hy co-activation. (C) When both PAG and Thal are entered into the mediation model as two separate mediators, only PAG is a complete mediator of the dmPFC–Hy co-activation. See Table 1 for abbreviations.
Similar articles
- Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions.
Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Kilts CD, et al. Neuroimage. 2003 Jan;18(1):156-68. doi: 10.1006/nimg.2002.1323. Neuroimage. 2003. PMID: 12507452 - Co-activation based parcellation of the human frontal pole.
Ray KL, Zald DH, Bludau S, Riedel MC, Bzdok D, Yanes J, Falcone KE, Amunts K, Fox PT, Eickhoff SB, Laird AR. Ray KL, et al. Neuroimage. 2015 Dec;123:200-11. doi: 10.1016/j.neuroimage.2015.07.072. Epub 2015 Aug 5. Neuroimage. 2015. PMID: 26254112 Free PMC article. - Neural correlates of successful emotional episodic encoding and retrieval: An SDM meta-analysis of neuroimaging studies.
Dahlgren K, Ferris C, Hamann S. Dahlgren K, et al. Neuropsychologia. 2020 Jun;143:107495. doi: 10.1016/j.neuropsychologia.2020.107495. Epub 2020 May 13. Neuropsychologia. 2020. PMID: 32416099 - Emotional pictures in the brain and their interaction with the task: A fine-grained fMRI coordinate-based meta-analysis study.
Mansueto SP, Romeo Z, Angrilli A, Spironelli C. Mansueto SP, et al. Neuroimage. 2025 Jan;305:120986. doi: 10.1016/j.neuroimage.2024.120986. Epub 2024 Dec 21. Neuroimage. 2025. PMID: 39716521 Review. - A Network Model of the Emotional Brain.
Pessoa L. Pessoa L. Trends Cogn Sci. 2017 May;21(5):357-371. doi: 10.1016/j.tics.2017.03.002. Epub 2017 Mar 28. Trends Cogn Sci. 2017. PMID: 28363681 Free PMC article. Review.
Cited by
- Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury.
Dennis M, Simic N, Bigler ED, Abildskov T, Agostino A, Taylor HG, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Yeates KO. Dennis M, et al. Dev Cogn Neurosci. 2013 Jul;5:25-39. doi: 10.1016/j.dcn.2012.11.006. Epub 2012 Nov 23. Dev Cogn Neurosci. 2013. PMID: 23291312 Free PMC article. - Abstract representations of associated emotions in the human brain.
Kim J, Schultz J, Rohe T, Wallraven C, Lee SW, Bülthoff HH. Kim J, et al. J Neurosci. 2015 Apr 8;35(14):5655-63. doi: 10.1523/JNEUROSCI.4059-14.2015. J Neurosci. 2015. PMID: 25855179 Free PMC article. - Encoding of reward expectation by monkey anterior insular neurons.
Mizuhiki T, Richmond BJ, Shidara M. Mizuhiki T, et al. J Neurophysiol. 2012 Jun;107(11):2996-3007. doi: 10.1152/jn.00282.2011. Epub 2012 Mar 7. J Neurophysiol. 2012. PMID: 22402653 Free PMC article. - States of mind: emotions, body feelings, and thoughts share distributed neural networks.
Oosterwijk S, Lindquist KA, Anderson E, Dautoff R, Moriguchi Y, Barrett LF. Oosterwijk S, et al. Neuroimage. 2012 Sep;62(3):2110-28. doi: 10.1016/j.neuroimage.2012.05.079. Epub 2012 Jun 5. Neuroimage. 2012. PMID: 22677148 Free PMC article. - A functional architecture of the human brain: emerging insights from the science of emotion.
Lindquist KA, Barrett LF. Lindquist KA, et al. Trends Cogn Sci. 2012 Nov;16(11):533-40. doi: 10.1016/j.tics.2012.09.005. Epub 2012 Oct 2. Trends Cogn Sci. 2012. PMID: 23036719 Free PMC article. Review.
References
Appendix A. Papers included in the meta-analysis
- Aalto S, Naatanen P, Wallius E, Metsahonkala L, Stenman H, Niem PM, et al. Neuroanatomical substrata of amusement and sadness: a PET activation study using film stimuli. Neuroreport. 2002;13(1):67–73. - PubMed
- Aalto S, Wallius E, Naatanen P, Hiltunen J, Metsahonkala L, Sipila H, et al. Regression analysis utilizing subjective evaluation of emotional experience in PET studies on emotions. Brain Research Protocols. 2005;15(3):142–154. - PubMed
- Adams RB, Jr, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300(5625):1536. - PubMed
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. - PubMed
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. - PubMed
References
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11(2):231–239. - PubMed
- Albus JS. A theory of cerebellar function. Math. Biosci. 1971;10:25–61.
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in Macaque monkeys. J. Comp. Neurol. 1998;401(4):455–479. - PubMed
- Aron AR, Fletcher PC, Bullmore T, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6:115–116. - PubMed
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004a;8(4):170–177. - PubMed
Publication types
MeSH terms
Grants and funding
- AG030311/AG/NIA NIH HHS/United States
- DP1OD003312/OD/NIH HHS/United States
- K02 MH001981/MH/NIMH NIH HHS/United States
- R01MH076136/MH/NIMH NIH HHS/United States
- DP1 OD003312/OD/NIH HHS/United States
- R01 AG030311/AG/NIA NIH HHS/United States
- R01 MH076136/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources