The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression - PubMed (original) (raw)
The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression
Hisaki Hayashi et al. J Biol Chem. 2008.
Abstract
Forkhead transcription factor Foxc2 is an essential regulator of the cardiovascular system in development and disease. However, the cellular and molecular functions of Foxc2 in vascular endothelial cells are still not fully understood. Here, through gene expression profiling in endothelial cells, we identified molecules associated with cell-extracellular matrix interactions, integrin beta3 (Itgb3), integrin beta5 (Itgb5), and fibronectin, as downstream targets of Foxc2. We found that Itgb3 expression is directly regulated by Foxc2 through multiple Forkhead-binding elements within two high homology regions in the Itgb3 promoter. Because Itgb3 is known to regulate angiogenesis, we further tested whether Foxc2 is directly involved in angiogenesis by regulating Itgb3 expression by in vitro experiments. Overexpression of Foxc2 significantly enhanced endothelial cell migration and adhesion, whereas this effect was strongly inhibited by Itgb3 neutralization antibody. In accordance with these results, pulmonary microvascular endothelial cells isolated from Foxc2 heterozygous mutant mice showed a marked reduction in Itgb3 expression and cell migration. Finally, ex vivo aortic ring assay to test the sprouting and microvessel formation revealed enhanced microvessel outgrowth by Foxc2 overexpression. Conversely, microvessel outgrowth from aortas of Foxc2 heterozygous mutant mice was reduced. Taken together, these results suggest that Foxc2 directly induces Itgb3 expression and regulates angiogenesis by Itgb3-mediated endothelial cell adhesion and migration.
Figures
FIGURE 1.
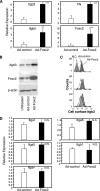
Foxc2 induces Itgb3 expression in endothelial cells. A, Foxc2 increases mRNA levels of Itgb3, Itgb5, and FN as detected by real-time RT-PCR. MEECs were infected with either Ad-control or Ad-Foxc2. Sixteen hours after infection, the cells were washed and cultured for 30 h. Total RNA was then isolated and subjected to real-time RT-PCR to detect expression levels of Itgb3, Itgb5, FN, and Foxc2. The experiment was independently repeated three times, and statistical significance was determined by Student's t tests. **, p < 0.01; ***,p < 0.005 versus Ad-control-infected cells. B, Foxc2 up-regulates Itgb3 protein in endothelial cells. Total protein lysates were prepared from MEECs infected with either Ad-Foxc2 or Ad-control and from untreated MEECs and subjected to Western blotting using antibodies against Foxc2 and Itgb3. Expression of β-actin is shown as a loading control.C, surface levels of Itgb3 expression in MEECs infected with either Ad-control or Ad-Foxc2 as measured by flow cytometry. Replicate analyses of three independent experiments are shown. The negative control (N.C.) indicates unstained MEECs. D, Foxc2 does not induce expression of_Itga1, Itga5, Itga6, Itgav_, and Itgb1. MEECs were infected with either Ad-control or Ad-Foxc2, and real-time RT-PCR was subsequently performed. The experiment was independently repeated three times, and statistical significance was determined by Student's t tests.N.S., non-significant.
FIGURE 2.
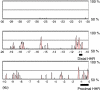
Alignment of 30-kb upstream regions from the transcription initiation site of Itgb3 between human and mouse using mVISTA. HHRs are shown in pink.
FIGURE 3.
Foxc2 regulates the proximal and distal promoters of Itgb3 in endothelial cells. A, schematic diagram of luciferase constructs for the proximal Itgb3 promoter (proximal HHR-Itgb3-LUC). Shown above are the mouse and human sequences for comparison. Note that the reported tandem repeats of the FBEs (highlighted) are located only in the mouse Itgb3 locus. B, Foxc2 activates the proximal_Itgb3_ promoter in a dose-dependent manner. MEECs were transfected with the Foxc2 expression vector together with either proximal HHR-Itgb3-LUC or its deletion construct, –793Itgb3-LUC. The -fold increase in normalized luciferase activity is shown. Statistical significance was determined by Student's t tests. ****, p < 0.001_versus_ the control. N.S., non-significant. C, schematic diagram of luciferase constructs for the distal Itgb3 promoter that consists of the three conserved FBEs (highlighted). Distal HHR-Itgb3-LUC (+P) and distal HHR-Itgb3-LUC (–P) indicate the presence and absence of the SV40 promoter in the constructs, respectively. Shown above are the mouse and human sequences for comparison. D, Foxc2 activates the distal Itgb3 promoter through two of the three FBEs. Luciferase assays were performed after transfection of MEECs with distal HHR-Itgb3-LUC or a series of mutant reporters (1Mut-LUC, 2Mut-LUC, 3Mut-LUC, and 1,2,3Mut-LUC) along with the Foxc2 expression vector. The -fold increase in normalized luciferase activity is shown. Data are presented as the means ± S.D. (n = 6) from two independent experiments. *, p < 0.05; **, p < 0.01 versus cells cotransfected with Foxc2 and pGL3-basic. E, Foxc2 binds to the FBEs in the distal or proximal HHRs in chromatin structure of endothelial cells. MEECs were subjected to chromatin immunoprecipitation assays using anti-Foxc2 antibody (Ab) or control IgG. Immunoprecipitated DNA was analyzed by PCR using primers specific to the proximal or distal HHR of mouse Itgb3.
FIGURE 4.
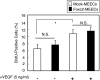
Proliferation assay for Foxc2-overexpressing endothelial cells. MEECs stably transfected with the mock or Foxc2 expression vector were cultured with or without VEGF (5 ng/ml) for 24 h in serum-reduced medium (0.5%) with a pulse of BrdUrd (BrdU) for the last 3 h. The cells were subsequently fixed and stained for BrdUrd and 4′,6-diamidino-2-phenylindole. The number of BrdUrd-positive cells and total cells was counted. Values are presented as the means ± S.D. from 10 microscopic fields from two independent experiments. Statistical significance was determined by Student's t tests. *, p < 0.05. N.S., non-significant.
FIGURE 5.
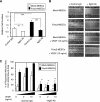
Increased adhesion and migration in Foxc2-overexpressing endothelial cells. A, Foxc2-induced cell adhesion is inhibited by blocking Itgb3 function. MEECs stably transfected with the mock or Foxc2 expression vector were preincubated with anti-Itgb3 antibody (Ab) or control IgG and plated on vitronectin-coated 96-well plates for 30 min. The relative numbers of the adherent cells were measured by absorbance after crystal violet staining. Data are presented as the means ± S.D. from three independent experiments. Statistical significance was determined by Student's t tests. **, p < 0.01; ***, p < 0.005. B, Foxc2-induced cell migration is inhibited by blocking Itgb3 function. Confluent MEECs stably transfected with the mock or Foxc2 expression vector were scraped to induce a wound and cultured with anti-mouse Itgb3 antibody or control IgG in the presence or absence of VEGF (10 ng/ml). At 24 h after wounding, the cells were stained with crystal violet and photographed.Unbroken and dotted lines define the wounded areas by the initial scraping and the recovered areas after 24 h, respectively. Cell migration was evaluated as described under “Materials and Methods.” Representative experiments are shown. C, quantitative measurement of cell migration during wound closure of B. Values are presented as the means ± S.D. of nine microscopic fields. The statistical significance of treatment with anti-Itgb3 antibody was determined by Student's t tests. *, p < 0.05; **, p < 0.01 versus corresponding mock- or Foxc2-overexpressing cells treated with control IgG.
FIGURE 6.
Foxc2 deficiency results in a reduction in endothelial cell migration and Itgb3 expression. A, reduced mRNA expression of Itgb3, Itgb5, and FN in PMVECs isolated from Foxc2+/– mice. Total RNA was prepared from PMVECs isolated from wild-type (WT) and Foxc2+/– mice, and real-time RT-PCR was performed. Results are presented as the means ± S.D. from triplicate experiments. Statistical significance was determined by Student's t tests. *, p < 0.05 versus the wild type. B, surface levels of Itgb3 expression in PMVECs isolated from wild-type and_Foxc2_+/– mice measured by flow cytometry. Replicate analyses of independently isolated PMVECs for each genotype are shown. Note the reduced expression of Itgb3 in Foxc2+/– cells compared with wild-type cells. C, no change in expression of_Itga1, Itga5, Itga6, Itgav_, and Itgb1 in_Foxc2_+/– PMVECs measured by real-time RT-PCR. Results are presented as the means ± S.D. from triplicate experiments. Statistical significance was determined by Student's t tests.N.S., non-significant. D, loss of Foxc2 does not affect endothelial cell proliferation. PMVECs isolated from wild-type and_Foxc2_+/– mice were cultured with or without VEGF (5 ng/ml) for 24 h in serum-reduced medium (0.5%) with a pulse of BrdUrd (BrdU) for the last 3 h. The number of BrdUrd-positive cells and total cells was counted. Values are presented as the means ± S.D. from 10 microscopic fields from two independent experiments. Statistical significance was determined by Student's t tests. *, p < 0.05. E, migration assay using PMVECs isolated from wild-type and_Foxc2_+/– mice. Primary PMVECs were added to Transwells coated with collagen I and allowed to migrate toward serum-free medium with or without VEGF (25 ng/ml). The number of migrating cells from randomly selected microscopic fields/well was counted at 5 h after cell plating. Data are the means ± S.D. from three experiments with eight data points for each. **, p < 0.01 versus the corresponding control.
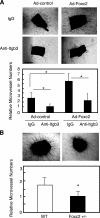
FIGURE 7.
Foxc2 regulates microvessel outgrowth through Itgb3 function from mouse aortic rings. A, aortic ring assay using wild-type aortas infected with Ad-Foxc2 or Ad-control. Thoracic aortas excised from wild-type (WT) mice were sagittally cut in two pieces, and a pair of equivalent tubes was infected with either Ad-Foxc2 or Ad-control. They were treated with either nonspecific control murine IgG or monoclonal antibody against Itgb3 at 20 mg/ml and then analyzed as described under “Materials and Methods”. B, aortic ring assay using_Foxc2_+/– mice. Thoracic aortas excised from_Foxc2_+/– mice were subjected to aortic ring assay as described above. Data are presented as the relative number of microvessels sprouting from aortic rings. Results are presented as the means ± S.D. (n = 9 or more). p values were determined by the corresponding sample indicated using Student's t test. *, p < 0.05 versus the corresponding control.
Similar articles
- Foxc2 transcription factor as a regulator of angiogenesis via induction of integrin beta3 expression.
Hayashi H, Kume T. Hayashi H, et al. Cell Adh Migr. 2009 Jan-Mar;3(1):24-6. doi: 10.4161/cam.3.1.7252. Epub 2009 Jan 22. Cell Adh Migr. 2009. PMID: 19372730 Free PMC article. - Endothelial immune activation programmes cell-fate decisions and angiogenesis by inducing angiogenesis regulator DLL4 through TLR4-ERK-FOXC2 signalling.
Xia S, Menden HL, Korfhagen TR, Kume T, Sampath V. Xia S, et al. J Physiol. 2018 Apr 15;596(8):1397-1417. doi: 10.1113/JP275453. Epub 2018 Mar 2. J Physiol. 2018. PMID: 29380370 Free PMC article. - Sprouty4 regulates endothelial cell migration via modulating integrin β3 stability through c-Src.
Gong Y, Yang X, He Q, Gower L, Prudovsky I, Vary CP, Brooks PC, Friesel RE. Gong Y, et al. Angiogenesis. 2013 Oct;16(4):861-75. doi: 10.1007/s10456-013-9361-x. Epub 2013 Aug 17. Angiogenesis. 2013. PMID: 23955631 Free PMC article. - Foxc2 transcription factor: a newly described regulator of angiogenesis.
Kume T. Kume T. Trends Cardiovasc Med. 2008 Aug;18(6):224-8. doi: 10.1016/j.tcm.2008.11.003. Trends Cardiovasc Med. 2008. PMID: 19185813 Free PMC article. Review. - Oncogenic functions of the FOXC2 transcription factor: a hallmarks of cancer perspective.
Hargadon KM, Goodloe TB 3rd, Lloyd ND. Hargadon KM, et al. Cancer Metastasis Rev. 2022 Dec;41(4):833-852. doi: 10.1007/s10555-022-10045-3. Epub 2022 Jun 14. Cancer Metastasis Rev. 2022. PMID: 35701636 Review.
Cited by
- Cell Transitions Contribute to Glucocorticoid-Induced Bone Loss.
Qiao X, Wu X, Zhao Y, Yang Y, Zhang L, Cai X, Ma JA, Ji J, Lyons K, Boström KI, Yao Y. Qiao X, et al. Cells. 2023 Jul 8;12(14):1810. doi: 10.3390/cells12141810. Cells. 2023. PMID: 37508475 Free PMC article. - Differential Gene Expression Profiling in Alveolar Echinococcosis Identifies Potential Biomarkers Associated With Angiogenesis.
Yimingjiang M, Aini A, Tuergan T, Zhang W. Yimingjiang M, et al. Open Forum Infect Dis. 2023 Jan 31;10(2):ofad031. doi: 10.1093/ofid/ofad031. eCollection 2023 Feb. Open Forum Infect Dis. 2023. PMID: 36817746 Free PMC article. - Dichotomous role of integrin-β5 in lung endothelial cells.
Blanchard N, Link PA, Farkas D, Harmon B, Hudson J, Bogamuwa S, Piper B, Authelet K, Cool CD, Heise RL, Freishtat R, Farkas L. Blanchard N, et al. Pulm Circ. 2022 Oct 1;12(4):e12156. doi: 10.1002/pul2.12156. eCollection 2022 Oct. Pulm Circ. 2022. PMID: 36438452 Free PMC article. - FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer.
Recouvreux MS, Miao J, Gozo MC, Wu J, Walts AE, Karlan BY, Orsulic S. Recouvreux MS, et al. Cancers (Basel). 2022 Oct 4;14(19):4851. doi: 10.3390/cancers14194851. Cancers (Basel). 2022. PMID: 36230774 Free PMC article. - FOXS1 Promotes Tumor Progression by Upregulating CXCL8 in Colorectal Cancer.
Qiu J, Li M, Su C, Liang Y, Ou R, Chen X, Huang C, Zhang Y, Ye Y, Liao W, Zhang C. Qiu J, et al. Front Oncol. 2022 Jul 8;12:894043. doi: 10.3389/fonc.2022.894043. eCollection 2022. Front Oncol. 2022. PMID: 35898871 Free PMC article.
References
- Carmeliet, P. (2005) Nature 438 932–936 - PubMed
- Serini, G., Valdembri, D., and Bussolino, F. (2006) Exp. Cell Res. 312 651–658 - PubMed
- Davis, G. E., and Senger, D. R. (2005) Circ. Res. 97 1093–1107 - PubMed
- Rupp, P. A., and Little, C. D. (2001) Circ. Res. 89 566–572 - PubMed
- Wilkinson-Berka, J. L., Jones, D., Taylor, G., Jaworski, K., Kelly, D. J., Ludbrook, S. B., Willette, R. N., Kumar, S., and Gilbert, R. E. (2006) Investig. Ophthalmol. Vis. Sci. 47 1600–1605 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL 067105/HL/NHLBI NIH HHS/United States
- HL 074121/HL/NHLBI NIH HHS/United States
- R01 EY019484/EY/NEI NIH HHS/United States
- R01 HL074121/HL/NHLBI NIH HHS/United States
- DK 068547/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous