Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation - PubMed (original) (raw)
Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation
Emma L Clayton et al. J Neurosci. 2008.
Abstract
Bulk endocytosis in central nerve terminals is activated by strong stimulation; however, the speed at which it is initiated and for how long it persists is still a matter of debate. To resolve this issue, we performed a characterization of bulk endocytic retrieval using action potential trains of increasing intensity. Bulk endocytosis was monitored by the loading of the fluorescent dyes FM2-10 and FM1-43, uptake of tetramethylrhodamine-dextran (40 kDa), or morphological analysis of uptake of the fluid-phase marker horseradish peroxidase. When neuronal cultures were subjected to mild stimulation (200 action potentials at 10 Hz), bulk endocytosis was not observed using any of our assay systems. However, when more intense trains of action potentials (400 or 800 action potentials at 40 and 80 Hz, respectively) were applied to neurons, bulk endocytosis was activated immediately, with the majority of bulk endocytosis complete by the end of stimulation. This contrasts with single synaptic vesicle endocytosis, the majority of which occurred after stimulation was terminated. Thus, bulk endocytosis is a fast event that is triggered during strong stimulation and provides the nerve terminal with an appropriate mechanism to meet the demands of synaptic vesicle retrieval during periods of intense synaptic vesicle exocytosis.
Figures
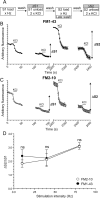
Figure 1.
No disparity in action potential-evoked poststimulation loading for FM1-43 and FM2-10. A, Granule neuron cultures were loaded with either FM1-43 or FM2-10 using trains of 200 (10 Hz), 400 (40 Hz), or 800 (80 Hz) action potentials. Dyes were loaded during stimulation at S1, but washout was delayed for 5 min at S2. Unloading was stimulated by 30 s stimuli of 50 m
m
KCl except for cultures loaded with 200 action potentials, for which two sequential trains of 400 action potentials (40 Hz) were used. B, C, Time course of the average response for nerve terminals loaded with either FM1-43 (B) or FM2-10 (C) during action potential stimulation at 80 Hz is displayed. D, Mean ΔS2/ΔS1 ratio ± SEM is displayed for each stimulation condition. All experiments were performed four times except those with 200 action potentials (n = 3). No significant difference between FM2-10 and FM1-43 loading was observed during any stimulation protocol (Student's t test).
Figure 2.
Uptake of large dextrans only occurs during strong action potential stimulation. Left, Representative fields in which tetramethylrhodamine-dextran (50 μ
m
) was applied to cultures during trains of 200 (A; 10 Hz), 400 (C; 40 Hz), or 800 (E; 80 Hz) action potentials and then immediately washed away. Right, Fields in which dextran was applied to cultures for 2 min immediately after trains of 200 (B; 10 Hz), 400 (D; 40 Hz), or 800 (F; 80 Hz) action potentials. Scale bar, 15 μm. Mean number of dextran puncta per field ± SEM is displayed for either during (G; open bars) or after (H; hatched bars) stimulation. Dotted line illustrates puncta number per field attributable to intrinsic background fluorescence in the absence of dextran. All experiments were performed three times. ***p < 0.001, one-way ANOVA.
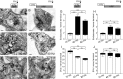
Figure 3.
HRP labels bulk endosomes when applied during strong action potential stimulation. A–F, HRP was applied to cultures during trains of 200 (A; 10 Hz), 400 (C; 40 Hz), or 800 (E; 80 Hz) action potentials and then immediately fixed. Alternatively, HRP was applied to cultures for 5 min immediately after trains of 200 (B; 10 Hz), 400 (D; 40 Hz), or 800 (F; 80 Hz) action potentials and then fixed. Representative electron micrographs are displayed. Black arrows indicate HRP-labeled endosomal structures, whereas white arrows indicate HRP-labeled SVs. Scale bars, 100 nm. Mean number ± SEM of either HRP-labeled (solid bars) or clear (open bars) endosomes (G) or SVs (I) generated during stimulation is displayed (200 action potentials, n = 17 nerve terminals; 400, n = 26; 800, n = 53). Mean number ± SEM of either HRP-labeled (solid bars) or clear (open bars) endosomes (H) or SVs (J) generated after stimulation is displayed per nerve terminal (200 action potentials, n = 18 nerve terminals; 400, n = 39; 800, n = 38). *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA for HRP structures.
Similar articles
- The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals.
Fernández-Alfonso T, Ryan TA. Fernández-Alfonso T, et al. Neuron. 2004 Mar 25;41(6):943-53. doi: 10.1016/s0896-6273(04)00113-8. Neuron. 2004. PMID: 15046726 - Activity-dependent bulk endocytosis and clathrin-dependent endocytosis replenish specific synaptic vesicle pools in central nerve terminals.
Cheung G, Jupp OJ, Cousin MA. Cheung G, et al. J Neurosci. 2010 Jun 16;30(24):8151-61. doi: 10.1523/JNEUROSCI.0293-10.2010. J Neurosci. 2010. PMID: 20554865 Free PMC article. - Quantitative monitoring of activity-dependent bulk endocytosis of synaptic vesicle membrane by fluorescent dextran imaging.
Clayton EL, Cousin MA. Clayton EL, et al. J Neurosci Methods. 2009 Dec 15;185(1):76-81. doi: 10.1016/j.jneumeth.2009.09.016. Epub 2009 Sep 17. J Neurosci Methods. 2009. PMID: 19766140 Free PMC article. - The synaptic vesicle: cycle of exocytosis and endocytosis.
Schweizer FE, Ryan TA. Schweizer FE, et al. Curr Opin Neurobiol. 2006 Jun;16(3):298-304. doi: 10.1016/j.conb.2006.05.006. Epub 2006 May 16. Curr Opin Neurobiol. 2006. PMID: 16707259 Review. - Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation.
Kononenko NL, Haucke V. Kononenko NL, et al. Neuron. 2015 Feb 4;85(3):484-96. doi: 10.1016/j.neuron.2014.12.016. Neuron. 2015. PMID: 25654254 Review.
Cited by
- The Active and Periactive Zone Organization and the Functional Properties of Small and Large Synapses.
Cano R, Tabares L. Cano R, et al. Front Synaptic Neurosci. 2016 May 24;8:12. doi: 10.3389/fnsyn.2016.00012. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 27252645 Free PMC article. - Actin- and dynamin-dependent maturation of bulk endocytosis restores neurotransmission following synaptic depletion.
Nguyen TH, Maucort G, Sullivan RK, Schenning M, Lavidis NA, McCluskey A, Robinson PJ, Meunier FA. Nguyen TH, et al. PLoS One. 2012;7(5):e36913. doi: 10.1371/journal.pone.0036913. Epub 2012 May 22. PLoS One. 2012. PMID: 22629340 Free PMC article. - Tissue-specific dynamin-1 deletion at the calyx of Held decreases short-term depression through a mechanism distinct from vesicle resupply.
Mahapatra S, Fan F, Lou X. Mahapatra S, et al. Proc Natl Acad Sci U S A. 2016 May 31;113(22):E3150-8. doi: 10.1073/pnas.1520937113. Epub 2016 May 16. Proc Natl Acad Sci U S A. 2016. PMID: 27185948 Free PMC article. - Adaptor protein complexes 1 and 3 are essential for generation of synaptic vesicles from activity-dependent bulk endosomes.
Cheung G, Cousin MA. Cheung G, et al. J Neurosci. 2012 Apr 25;32(17):6014-23. doi: 10.1523/JNEUROSCI.6305-11.2012. J Neurosci. 2012. PMID: 22539861 Free PMC article. - Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1.
Lou X, Paradise S, Ferguson SM, De Camilli P. Lou X, et al. Proc Natl Acad Sci U S A. 2008 Nov 11;105(45):17555-60. doi: 10.1073/pnas.0809621105. Epub 2008 Nov 5. Proc Natl Acad Sci U S A. 2008. PMID: 18987309 Free PMC article.
References
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. - PubMed
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. - PubMed
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources