The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes - PubMed (original) (raw)
The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes
Yiying Cai et al. Cell. 2008.
Abstract
The multimeric membrane-tethering complexes TRAPPI and TRAPPII share seven subunits, of which four (Bet3p, Bet5p, Trs23p, and Trs31p) are minimally needed to activate the Rab GTPase Ypt1p in an event preceding membrane fusion. Here, we present the structure of a heteropentameric TRAPPI assembly complexed with Ypt1p. We propose that TRAPPI facilitates nucleotide exchange primarily by stabilizing the nucleotide-binding pocket of Ypt1p in an open, solvent-accessible form. Bet3p, Bet5p, and Trs23p interact directly with Ypt1p to stabilize this form, while the C terminus of Bet3p invades the pocket to participate in its remodeling. The Trs31p subunit does not interact directly with the GTPase but allosterically regulates the TRAPPI interface with Ypt1p. Our findings imply that TRAPPII activates Ypt1p by an identical mechanism. This view of a multimeric membrane-tethering assembly complexed with a Rab provides a framework for understanding events preceding membrane fusion at the molecular level.
Figures
Figure 1
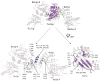
Structure of Ypt1p bound to TRAPPI subcomplex. (A) The TRAPPI complex is shown as a surface representation, and Ypt1p is shown as a backbone worm. In TRAPPI, different polypeptide chains are colored differently. In Ypt1p, switch regions I and II are yellow. At left, the positions of Cys80 and the hydrophobic channels in the two copies of Bet3p are labeled with red arrows. The right panel shows the same complex rotated about the horizontal axis indicated. (B) A surface representation of TRAPPI colored by electrostatic potential (blue=basic; red=acidic), with Ypt1p shown as a backbone worm. The orientations of the protein complexes are identical in panels A and B. (C) A ribbons representation of TRAPPI oriented as in panel A, left. The palmitoylate group modeled into the Bet3p-A hydrophobic channel is yellow.
Figure 2
The Ypt1p/TRAPPI interface. At top, the complex is shown with Ypt1p in purple, except for switches I and II (orange) and the P-loop (yellow). The TRAPPI complex is white, except for the C-terminus of Bet3p-A (green). At bottom, Ypt1p has been pulled away from the TRAPPI surface and rotated by 180 ° about a vertical axis. Cα positions of residues within 4 Å of the interface are labeled and colored.
Figure 3
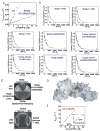
The mechanism for nucleotide exchange. Comparison of the nucleotide-free form of Ypt1p in the Ypt1p/TRAPPI complex with (A) GDP-bound Rab1a (PDB ID 2FOL) and (B) GppNHp-bound Ypt1p (1YZN). The nucleotide-free form of Ypt1p is purple, except for the switch regions and the P-loop (lavender). The C-terminus of Bet3p-A, including the E192 and D193 side chains, is green. Nucleotide-bound forms of Ypt1p/Rab1a are white, including the Y33 side chain. Nucleotide is grey and the magnesium cation blue. (C) GDP dissociates from the Ypt1p (Y33A) mutant ~10 times faster than from wt Ypt1p. Time course of [3H]-GDP dissociation from 400 nM Ypt1p (black) and Ypt1p(Y33A) (green) is shown; it was measured by a filter binding assay described in the Supplemental Methods. Solid line through the data is the best fit to a single exponential yielding the GDP dissociation rate constant (_k_−GDP) of 1.21±0.22 ×10−4 s−1 for Ypt1p and 16.3±0.6 ×10−4 for Ypt1p(Y33A). The inset shows the same data over a shorter time interval. (D) GDP associates with Ypt1p and Ypt1p(Y33A) at similar rates. MantGDP binding to nucleotide-free Ypt1p or Ypt1p(Y33A) was continuously monitored by fluorescence (Figure S6A). The concentration dependence of _k_obs for Ypt1p (black) and Ypt1p(Y33A) (green) is shown. The second-order association rate constant for GDP binding (k+GDP) determined from the slopes of the best fits to the data over this range are 0.05±0.01 μM−1 s−1 for Ypt1p and 0.07±0.01 μM−1 s−1 for Ypt1p(Y33A).
Figure 4
Kinetic and mutational analysis of TRAPPI. Data in (A–B) were used in calculating “kcat/KM” and _k_−GDP,obs values reported in Table 1. (A) MantGDP dissociation from Ypt1p (250 nM) in the presence of TRAPPI (Bet3p, Bet5p, Trs20p, Trs23p, and Trs31p) and TRAPPI mutants was continuously monitored by fluorescence (λex=280 nm, λem=435 nm; Figure S6C). TRAPPI (red) and Bet3p (E192A/D193A) mutant (blue) concentration dependence of the observed GDP dissociation rate constant (_k_−mGDP,obs) is shown; filter binding assays are described in the Supplemental Methods. The solid lines are best fits of the data to linear functions, yielding the catalytic efficiencies (apparent kcat/KM) from the slopes. (B) Other mutants had only residual GEF activities. Time courses of [3H]-GDP dissociation from 400 nM Ypt1p in the absence (black) or presence (blue) of the indicated TRAPPI mutant are shown; Table 1 lists TRAPPI concentrations. Solid lines are the best fits to single exponentials yielding the observed GDP dissociation rate constant (_k_−GDP,obs). (C) Yeast cells harboring point mutations in bet5 and trs23, which reduce GEF activity in vitro, were grown at 25°C on 5-FOA plates. All these mutants were inviable, except for trs23 (H41A/G42M/A45W/I46R), which also grows at 37°C (data not shown). Yeast cells harboring C-terminal truncations in bet3, which reduce GEF activity in vitro, were grown at 25 °C and 37 °C on YPD plates. The 37 °C plate is shown. The bet3 truncation mutants were viable. (D) TRAPPI oriented as in Figure 1A, left. Residues that were mutated are indicated. Green: residues 192–193 in Bet3p. Cyan: 46, 50 in Bet5p; Orange: 12, 14, 34 in Trs23p; Purple: 200–201, 203 in Trs23p.Yellow: 38 in Trs23p. Indigo:41–42, 45–46 in Trs23p. (E) TRAPPI-bound Ypt1p associates with GTP ~30 times faster than Ypt1p alone. MantGTP binding to nucleotide-free Ypt1p or Ypt1p/TRAPPI complex was monitored by fluorescence (Figure S6B). MantGTP concentration dependence of _k_obs for Ypt1p (black) and Ypt1p/TRAPPI complex (red) is shown. The second-order association rate constant for mantGTP binding (k+GTP) determined from the slopes of the best fits of the data are 0.10±0.01 μM−1 s−1 for Ypt1p and 3.2±0.1 μM−1 s−1 for Ypt1p/TRAPPI.
Figure 5
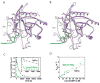
A superposition of the yeast TRAPPI subcomplex containing Trs31p/Bet3p-B/Trs23p/Bet5p/Bet3p-A with the Trs20/Trs31/Bet3-B (yellow, PDB ID 2J3W) and Trs23/Bet5/Bet3-A/Trs33 (white, 2J32) complexes from vertebrates. A PDZ-like domain is present only in mammalian Trs23. Yeast proteins are colored as in Figure 1, (A) and (C), and the complex is oriented as in panels A–C, left. The largest deviations between the yeast and vertebrate assemblies are at the 3-way interface between Trs23p, Trs31p, and Bet3p-B (arrow). (B) Model for the mammalian TRAPP core as it tethers two COPII coated vesicles via interactions between Bet3 subunits of TRAPP and Sec23 of the COPII coat. The two copies of Bet3 and the two vesicles are related by the same 2-fold rotation about an axis perpendicular to the plane of the page. Equivalent surface patches on Bet3-A and –B are accessible to both vesicles only if TRAPP is oriented as shown. The C-terminal residues (175–206) absent from Ypt1p (purple) in our structure could span the ~40 Å to the membrane. Ypt1p/Rab1a becomes anchored in the membrane by its prenylated C-terminus.
Figure 6
Yeast TRAPPII potently stimulates the GEF activity of Ypt1p, but not Ypt31p or Ypt32p. (A) Protein A tagged TRAPPI and TRAPPII were prepared as described in Supplemental Methods. The GEF activity on Ypt1p, stimulated by TRAPPI and TRAPPII, was normalized to Trs33p. The assay is described in the Supplemental Methods. The data shown were obtained from four separate experiments. Error bars are SEM. (B) Equivalent amounts of recombinant GST, GST-Ypt1p-GDP and GST-Ypt1p-GTP were loaded with nucleotide as described in Supplemental Methods, incubated with yeast lysate as before (Wang et al., 2000), and blotted for the TRAPPII-specific subunit Trs130p. (C) The TRAPPII complex, isolated as described above, was assayed for its ability to stimulate nucleotide exchange on Ypt31p and (D) Ypt32p. For comparison, an equivalent amount of TRAPPII was assayed for its ability to activate Ypt1p. The amount of TRAPPII assayed in (C) and (D) was not equivalent. Error bars are SEM.
Comment in
- Team effort by TRAPP forces a nucleotide fumble.
Nottingham RM, Pfeffer SR. Nottingham RM, et al. Cell. 2008 Jun 27;133(7):1141-3. doi: 10.1016/j.cell.2008.06.012. Cell. 2008. PMID: 18585348
Similar articles
- The TRAPP complexes: discriminating GTPases in context.
Bagde SR, Fromme JC. Bagde SR, et al. FEBS Lett. 2023 Mar;597(6):721-733. doi: 10.1002/1873-3468.14557. Epub 2022 Dec 21. FEBS Lett. 2023. PMID: 36481981 Free PMC article. Review. - Team effort by TRAPP forces a nucleotide fumble.
Nottingham RM, Pfeffer SR. Nottingham RM, et al. Cell. 2008 Jun 27;133(7):1141-3. doi: 10.1016/j.cell.2008.06.012. Cell. 2008. PMID: 18585348 - Trs65p, a subunit of the Ypt1p GEF TRAPPII, interacts with the Arf1p exchange factor Gea2p to facilitate COPI-mediated vesicle traffic.
Chen S, Cai H, Park SK, Menon S, Jackson CL, Ferro-Novick S. Chen S, et al. Mol Biol Cell. 2011 Oct;22(19):3634-44. doi: 10.1091/mbc.E11-03-0197. Epub 2011 Aug 3. Mol Biol Cell. 2011. PMID: 21813735 Free PMC article. - TRAPP stimulates guanine nucleotide exchange on Ypt1p.
Wang W, Sacher M, Ferro-Novick S. Wang W, et al. J Cell Biol. 2000 Oct 16;151(2):289-96. doi: 10.1083/jcb.151.2.289. J Cell Biol. 2000. PMID: 11038176 Free PMC article. - The TRAPP complexes: oligomeric exchange factors that activate the small GTPases Rab1 and Rab11.
Galindo A, Munro S. Galindo A, et al. FEBS Lett. 2023 Mar;597(6):734-749. doi: 10.1002/1873-3468.14553. Epub 2022 Dec 18. FEBS Lett. 2023. PMID: 36477798 Free PMC article. Review.
Cited by
- Comparative genomic analysis and phylogeny of NAC25 gene from cultivated and wild Coffea species.
Huded AKC, Jingade P, Mishra MK, Ercisli S, Ilhan G, Marc RA, Vodnar D. Huded AKC, et al. Front Plant Sci. 2022 Sep 16;13:1009733. doi: 10.3389/fpls.2022.1009733. eCollection 2022. Front Plant Sci. 2022. PMID: 36186041 Free PMC article. - The TRAPP complexes: discriminating GTPases in context.
Bagde SR, Fromme JC. Bagde SR, et al. FEBS Lett. 2023 Mar;597(6):721-733. doi: 10.1002/1873-3468.14557. Epub 2022 Dec 21. FEBS Lett. 2023. PMID: 36481981 Free PMC article. Review. - TRAPP complexes in membrane traffic: convergence through a common Rab.
Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. Barrowman J, et al. Nat Rev Mol Cell Biol. 2010 Nov;11(11):759-63. doi: 10.1038/nrm2999. Nat Rev Mol Cell Biol. 2010. PMID: 20966969 - Entry and exit mechanisms at the cis-face of the Golgi complex.
Lorente-Rodríguez A, Barlowe C. Lorente-Rodríguez A, et al. Cold Spring Harb Perspect Biol. 2011 Jul 1;3(7):a005207. doi: 10.1101/cshperspect.a005207. Cold Spring Harb Perspect Biol. 2011. PMID: 21482742 Free PMC article. Review. - Tip20p reaches out to Dsl1p to tether membranes.
Munson M. Munson M. Nat Struct Mol Biol. 2009 Feb;16(2):100-2. doi: 10.1038/nsmb0209-100. Nat Struct Mol Biol. 2009. PMID: 19190660 Free PMC article.
References
- Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343. - PubMed
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. - PubMed
- Brunger AT, et al. Crystallography and NMR system (CNS): a new software suite for macromolecular structure determination. Acta Cryst. 1998;D54:904–921. - PubMed
- Cai H, Reinisch K, Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007a;12:671–82. - PubMed
- Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007b;445:941–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038041/NS/NINDS NIH HHS/United States
- GM071688/GM/NIGMS NIH HHS/United States
- R01 DA002243/DA/NIDA NIH HHS/United States
- T32-GM07223/GM/NIGMS NIH HHS/United States
- R56 NS038041/NS/NINDS NIH HHS/United States
- RR020171/RR/NCRR NIH HHS/United States
- R01 GM071688/GM/NIGMS NIH HHS/United States
- F31 DC009143/DC/NIDCD NIH HHS/United States
- P20 RR020171/RR/NCRR NIH HHS/United States
- 1F31DC009143-01/DC/NIDCD NIH HHS/United States
- R01 NS038041-04/NS/NINDS NIH HHS/United States
- R01 GM071688-02/GM/NIGMS NIH HHS/United States
- DA02243/DA/NIDA NIH HHS/United States
- R01 GM080616/GM/NIGMS NIH HHS/United States
- F31 DC009143-01/DC/NIDCD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- GM080616/GM/NIGMS NIH HHS/United States
- R01 GM080616-01/GM/NIGMS NIH HHS/United States
- NS38041/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases