NOD-like receptors (NLRs): bona fide intracellular microbial sensors - PubMed (original) (raw)
Review
NOD-like receptors (NLRs): bona fide intracellular microbial sensors
Michael H Shaw et al. Curr Opin Immunol. 2008 Aug.
Abstract
The nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) (nucleotide-binding domain leucine-rich repeat containing) family of proteins has been demonstrated to function as regulators of innate immune response against microbial pathogens. Stimulation of NOD1 and NOD2, two prototypic NLRs, results in the activation of MAPK and NF-kappaB. On the other hand, a different set of NLRs induces caspase-1 activation through the assembly of an inflammasome. This review discusses recent findings regarding the signaling pathways utilized by NLR proteins in the control of caspase-1 and NF-kappaB activation, as well as the nonredundant role of NLRs in pathogen clearance. The review also covers advances regarding the cellular localization of these proteins and the implications this may have on pathogen sensing and signal transduction.
Figures
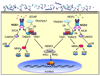
Figure 1. NOD1/NOD2 activation and signaling
NOD1 and NOD2 sense peptidoglycan-derived motifs in the cytosol and form a complex with RICK. K63-linked regulatory ubiquitination of RICK leads to the recruitment of TAK1. The activation and recruitment of TAK1 complex to RICK further recruits the IKK complex. The interaction between RICK and NEMO ultimately results in the activation of IKKs and MKK by TAK1.
Figure 2. Cellular localization of NOD1 and NOD2
A) During S. flexneri infection NOD1 is recruited to the bacterial entry site. It is unclear whether NOD1 is recruited to the bacterial contact foci from the cytosol or the plasma membrane fraction. Nevertheless, the co-localization of NOD1 with the bacterial contact point suggests that PGN sensing and signaling by NOD1 occurs at this initial site of contact. B) MDP is internalized into an acidified endosomal compartment. NOD2 could be sensing MDP at the plasma membrane and becomes associated with the vesicle following invagination of the plasma membrane during vesicle formation (1). Alternatively, NOD2 could be actively recruited to the MDP-containing vesicle after internalization (2). The localization of MDP to vesicle suggests that NOD2 signaling may be emanating from these vesicular structures. C) NLRX1, a mitochondria-targeted NLR protein, might act as a negative regulator of MAVS signalling, diminishing virally induced RIG-like helicase interactions. In a different model, NLRX1 promotes ROS production at the mitochondria, which consequently helps to fight bacteria and viruses. How NLRX1 is activated remains unclear.
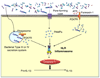
Figure 3. Productive IL-1β secretion is a two-step process
TLR signaling induces the synthesis of pro-IL-1β. Bacteria and/or bacterial products enter the cytosol either through the actions of pore-forming toxins or ATP-mediated activation of the pannexin-1 pore. Activation of NLRs by cytosolic PAMPs promotes the formation of caspase-1 activating ‘inflammasome’. Active caspase-1 then processes the IL-1β precursor into the bioactive form for secretion.
Similar articles
- Mutational analysis of human NOD1 and NOD2 NACHT domains reveals different modes of activation.
Zurek B, Proell M, Wagner RN, Schwarzenbacher R, Kufer TA. Zurek B, et al. Innate Immun. 2012 Feb;18(1):100-11. doi: 10.1177/1753425910394002. Epub 2011 Feb 10. Innate Immun. 2012. PMID: 21310790 - Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases.
Correa RG, Milutinovic S, Reed JC. Correa RG, et al. Biosci Rep. 2012 Dec;32(6):597-608. doi: 10.1042/BSR20120055. Biosci Rep. 2012. PMID: 22908883 Free PMC article. Review. - Intracellular NOD-like receptors in innate immunity, infection and disease.
Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Núñez G. Franchi L, et al. Cell Microbiol. 2008 Jan;10(1):1-8. doi: 10.1111/j.1462-5822.2007.01059.x. Epub 2007 Oct 18. Cell Microbiol. 2008. PMID: 17944960 Review. - Intracellular NOD-like receptors in host defense and disease.
Kanneganti TD, Lamkanfi M, Núñez G. Kanneganti TD, et al. Immunity. 2007 Oct;27(4):549-59. doi: 10.1016/j.immuni.2007.10.002. Immunity. 2007. PMID: 17967410 Review. - The Nodosome: Nod1 and Nod2 control bacterial infections and inflammation.
Tattoli I, Travassos LH, Carneiro LA, Magalhaes JG, Girardin SE. Tattoli I, et al. Semin Immunopathol. 2007 Sep;29(3):289-301. doi: 10.1007/s00281-007-0083-2. Epub 2007 Aug 10. Semin Immunopathol. 2007. PMID: 17690884 Review.
Cited by
- Nod-Like Receptors in Host Defence and Disease at the Epidermal Barrier.
Danis J, Mellett M. Danis J, et al. Int J Mol Sci. 2021 Apr 28;22(9):4677. doi: 10.3390/ijms22094677. Int J Mol Sci. 2021. PMID: 33925158 Free PMC article. Review. - Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells.
Moranta D, Regueiro V, March C, Llobet E, Margareto J, Larrarte E, Garmendia J, Bengoechea JA. Moranta D, et al. Infect Immun. 2010 Mar;78(3):1135-46. doi: 10.1128/IAI.00940-09. Epub 2009 Dec 14. Infect Immun. 2010. PMID: 20008534 Free PMC article. - Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases.
Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Shio MT, et al. PLoS Pathog. 2009 Aug;5(8):e1000559. doi: 10.1371/journal.ppat.1000559. Epub 2009 Aug 21. PLoS Pathog. 2009. PMID: 19696895 Free PMC article. - Giardia cyst wall protein 1 is a lectin that binds to curled fibrils of the GalNAc homopolymer.
Chatterjee A, Carpentieri A, Ratner DM, Bullitt E, Costello CE, Robbins PW, Samuelson J. Chatterjee A, et al. PLoS Pathog. 2010 Aug 19;6(8):e1001059. doi: 10.1371/journal.ppat.1001059. PLoS Pathog. 2010. PMID: 20808847 Free PMC article. - Transcriptional profiling of hilar nodes from pigs after experimental infection with Actinobacillus pleuropneumoniae.
Yu S, Zuo Z, Cui H, Li M, Peng X, Zhu L, Zhang M, Li X, Xu Z, Gan M, Deng J, Fang J, Ma J, Su S, Wang Y, Shen L, Ma X, Ren Z, Wu B, Hu Y. Yu S, et al. Int J Mol Sci. 2013 Nov 29;14(12):23516-32. doi: 10.3390/ijms141223516. Int J Mol Sci. 2013. PMID: 24351863 Free PMC article.
References
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. - PubMed
- Inohara, Chamaillard, McDonald C, Nuñez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. - PubMed
- Derry WB, Putzke AP, Rothman JH. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. - PubMed
- Liu QA, Hengartner MO. The molecular mechanism of programmed cell death in C. elegans. Ann N Y Acad Sci. 1999;887:92–104. - PubMed
- Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–27831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR052756-03/AR/NIAMS NIH HHS/United States
- R01 AR052756/AR/NIAMS NIH HHS/United States
- R01 AI064748-04/AI/NIAID NIH HHS/United States
- R01 AI063331/AI/NIAID NIH HHS/United States
- R01 AI063331-04/AI/NIAID NIH HHS/United States
- R01 AI064748/AI/NIAID NIH HHS/United States
- R01 DK061707-07/DK/NIDDK NIH HHS/United States
- R01 DK061707/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources