PAK is regulated by PI3K, PIX, CDC42, and PP2Calpha and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway - PubMed (original) (raw)
PAK is regulated by PI3K, PIX, CDC42, and PP2Calpha and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway
Perry M Chan et al. J Biol Chem. 2008.
Abstract
Fractionation of brain extracts and functional biochemical assays identified PP2Calpha, a serine/threonine phosphatase, as the major biochemical activity inhibiting PAK1. PP2Calpha dephosphorylated PAK1 and p38, both of which were activated upon hyperosmotic shock with the same kinetics. In comparison to growth factors, hyperosmolality was a more potent activator of PAK1. Therefore we characterize the PAK signaling pathway in the hyperosmotic shock response. Endogenous PAKs were recruited to the p38 kinase complex in a phosphorylation-dependent manner. Overexpression of a PAK inhibitory peptide or dominant negative Cdc42 revealed that p38 activation was dependent on PAK and Cdc42 activities. PAK mutants deficient in binding to Cdc42 or PAK-interacting exchange factor were not activated. Using a panel of kinase inhibitors, we identified PI3K acting upstream of PAK, which correlated with PAK repression by pTEN overexpression. RNA interference knockdown of PAK expression reduced stress-induced p38 activation and conversely, PP2Calpha knockdown increased its activation. Hyperosmotic stress-induced PAK translocation away from focal adhesions to the perinuclear compartment and resulted in disassembly of focal adhesions, which are hallmarks of PAK activation. Inhibition of PAK by overexpression of PP2Calpha or the kinase inhibitory domain prevented sorbitol-induced focal adhesion dissolution. Inhibition of MAPK pathways showed that MEK-ERK signaling but not p38 is required for full PAK activation and focal adhesion turnover. We conclude that 1) PAK plays a required role in hyperosmotic signaling through the PI3K/pTEN/Cdc42/PP2Calpha/p38 pathway, and 2) PAK and PP2Calpha modulate the effects of this pathway on focal adhesion dynamics.
Figures
FIGURE 1.
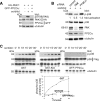
Identification and characterization of PP2Cα as the major phosphatase of PAK1 in brain lysate. A, detection of PAK1 inhibitory activity in rat brain lysate. Recombinant GST-PAK1 (0.24 mg/ml) was used in a kinase assay with native or heat-treated (68 °C for 10 min) lysates, or an equal amount of bovine serum albumin (BSA) for control (0.1 mg/ml). The kinase reactions were incubated at 30 °C for 10 min in the presence of [γ-32P]ATP and terminated by adding SDS-sample buffer and boiling for 2 min. Following SDS-PAGE, incorporated32P-labeled phosphate was visualized by autoradiography (top panel); phosphorylation of PAK1 resulted in its decreased mobility (bottom panel). B, characterization of phosphatases in brain lysate acting on PAK1. PAK phosphatase activity in lysate was assayed in the presence of proteinserine/threonine phosphatase inhibitors okadaic acid (0.1 μ
m
), sodium fluoride (2 m
m
), orimidazole (1 m
m
). Divalent cation dependence of the phosphatase was tested using MgCl2 (10 m
m
), MnCl2 (1 m
m
), and the cation chelator EDTA (50 m
m
). Okadaic acid and microcystin showed inhibitory activity only at concentrations higher than 2 μ
m
and 200 n
m
, respectively. C, scheme of biochemical fractionation and PP2Cα immunodepletion of brain lysate. Fractions separated by mono-S chromatography that contained phosphatase activity on PAK1 (supplemental Fig. 1) were pooled and passed through a column containing anti-PP2Cα antibody immobilized on protein A-Sepharose. The depleted fraction (flow-through) and pre-depleted lysate in equal amounts were compared for PAK1 phosphatase activity. Western blotting of crude brain extract with the anti-PP2Cα antibody showed a single band at 42 kDa (data not shown). The flow-through, wash, and bound fractions were analyzed for the presence of protein serine/threonine phosphatases PP2A, PP2B, POPX1, and POPX2 by Western blotting. D, comparison of PP2Cα, PP1, and PP2A phosphatase activities on pre-activated PAK1. The three phosphatases were used in equal molar concentrations (0.9 μ
m
) and dephosphorylation of PAK1 was followed by Western blotting for Ser(P)57 and Ser(P)198, two known PAK phosphosites. Dephosphorylation of PAK1 was most efficiently performed by PP2Cα (curves a and b), followed by PP2A (e and_f_), and last, PP1 (c and d). The t½ (reaction time required to dephosphorylate 50% of phospho-protein) and _R_2 values were obtained by statistical curve fitting of densitometric values. E, comparison of PAK isoforms and other protein serine/threonine kinases as substrates for PP2Cα. The recombinant protein kinases were purified to >95%, pre-phosphorylated in the presence of [γ-32P]ATP and used at the same concentration of 24 μg/ml. PP2Cα was added to a concentration of 0.05 μg/ml to initiate the reaction. PP2Cα most efficiently dephosphorylated full-length PAK1 and PAK2, followed by αMRCK and βMRCK catalytic domains and finally PKCα catalytic domain.
FIGURE 2.
Characterization of PAK1 and p38 in hyperosmotic shock. A, sorbitol is a potent activator of PAK1. COS7 cells were transfected with HA-PAK1, and treated with or without sorbitol for 30 min, or serum (10%), epidermal growth factor, or platelet-derived growth factor (100 ng/ml) for 10 min. Levels of phospho-PAK1 were quantitated by densitometric analysis of Western blots. Phospho-PAK1 in untreated cells was standardized at the value of 1 and other treatment conditions were normalized to that value. Mean values of two independent assays are labeled above the bars. Statistical analysis using one-way analysis of variance yielded the p value of 0.004. B, kinetics of PAK1 and p38 activation align closely under high osmolarity. In COS7 cells overexpressing HA-PAK1 and FLAG-p38, treatment with 0.8
m
sorbitol resulted in similar kinetics for PAK1 and p38 activation, as assessed by phospho-PAK1 and phospho-p38 Western blotting.C, PAK is activated and recruited to the p38 complex under high osmolarity. In HeLa cells transfected with FLAG-tagged p38, endogenous PAKs was activated (as detected by PAK phospho-specific antibodies; residue numbers according to human PAK) and recruited to the phospho-p38 complex at high concentrations of sorbitol. Activation of JNK, p38, and ERK was proportional to increasing concentrations of sorbitol, as probed by phospho-specific MAPK antibodies. PAK did not co-precipitate with phospho-JNK or phospho-ERK under the conditions used (data not shown). M2 IP and hc represent M2 anti-FLAG immunoprecipitation complexes and IgG heavy chain, respectively.D, overexpression of group I PAKs can augment hyperosmotic-induced p38 phosphorylation. Cells were transfected with PAK1, -2, -3, or dominant active Cdc42, alone or together with PP2Cα, and treated with sorbitol. Endogenous phospho-p38 was elevated by overexpression of PAKs or Cdc4212V, and decreased by PP2Cα. Levels of phospho-p38 was quantitated by densitometry and normalized to the p38 response in untransfected cells. E, constitutively active PAK1 co-precipitated with the p38 complex following hyperosmotic shock. COS7 cells co-transfected with FLAG-p38 and wild-type, L107F (constitutively active), or L107F/T422A (open conformation, non-activable) versions of PAK1 were treated with 0.8
m
sorbitol for 30 min. The constitutively active PAK1 displayed higher affinity than the wild-type kinase for the p38 complex.
FIGURE 3.
Characterization of PAK1 and p38 regulation by PP2Cαphosphatase in the hyperosmotic response. A, overexpression of PP2Cα phosphatase down-regulated both PAK1 and endogenous p38. COS7 cells transfected with FLAG-tagged wild-type PAK1, with or without PP2Cα, were treated with sorbitol for 30 min. Levels of both phospho-PAK1 and endogenous phospho-p38 were decreased in the presence of elevated phosphatase. B, effects of RNAi knockdown of PAK and PP2Cα on p38 phosphorylation. N1E-115 cells transfected with siRNA targeting PAK (resulting in 75% knockdown) displayed reduced stress activation of p38 to ∼50% of normal, and cells transfected with siRNA targeting PP2Cα (50% knockdown) up-regulated p38 to 130% above normal. The control siRNA directed against GFP had no effect. N1E-115 cells were used because endogenous levels of PAK and PP2Cα are easily detectable. C, the inhibition of PAK versus PP2Cα overexpression was compared for the efficacy and duration of p38 inhibition. COS7 cells were transfected with FLAG-p38 together with GST, GST-KID (PAK1 kinase inhibitory domain peptide; residues 83-149), or GFP-PP2Cα for 24 h before sorbitol treatment. Overexpression of the phosphatase had a stronger effect on reducing p38 phosphorylation than inhibition of PAK.
FIGURE 4.
Role of Rho GTPases and binding requirements for PAK1 and p38 signaling. A, the role of Cdc42, Rac, and RhoA on osmoinduced PAK1 activation was examined. COS7 cells were transfected with dominant-negative versions of the Rho GTPases and FLAG-PAK1. Levels of PAK1 activity were assessed for cells with or without sorbitol treatment by immunoprecipitating PAK1 and assessing autophosphorylation of the kinase in the presence of [32P]ATP for 10 min at 30 °C. This showed that interfering with Cdc42 strongly reduced sorbitol activation of PAK1. The basal levels of PAK1 in untreated cells for each Rho GTPase were standardized to 1. PAK fold activation was measured by densitometric analyses of autoradiograms.B, overexpression of GST-KID peptide or dominant-negative Cdc42 inhibited activation of p38 but not of JNK. COS7 cells were transfected with FLAG-p38 or FLAG-JNK alone, or cotransfected with GST-KID or HA-Cdc4217N before sorbitol treatment. C, role of PAK1-interacting partners in kinase activation. PAK mutants defective in binding specific proteins were used: S76P, defective in Cdc42 binding; P191G/R192A, defective in PIX binding; Δ_22N.T_. (NH2 terminus), defective in NCK binding;S198/203A and T422A, negative controls as non-activable kinases. FLAG-PAK wild-type and mutants were transfected and analyzed for osmo-induced activity as in A. PAK mutants defective in Cdc42 and PIX binding consistently showed slower mobility than the wild-type kinase, despite not being activated in these assays. D, PI3K/Cdc42/PAK signaling pathway leads to stress-induced p38 phosphorylation. COS7 cells were transfected with FLAG-PAK1 and FLAG-p38 and treated with the indicated drugs 2 h prior to osmotic shock. DMSO (no drug) was used as the control and normalized to 100% of phosphorylated kinase. Phosphorylated PAK and p38 were assessed by Western blotting and quantitated by densitometry. The PI3K inhibitors LY294002 and wortmannin significantly inhibited both PAK and p38 phosphorylation. Statistical analyses using one-way analysis of variance yielded a p value of 0.002 for phospho-PAK assays and a p value of 0.012 for phospho-p38 assays. E, COS7 cells were transfected with FLAG-PAK1 alone or cotransfected with GST-KID, GFP-PP2Cα, HA-pTENWT, or HA-pTENC124S (inactive phosphatase) and subjected to osmotic shock. Overexpression of pTENWT but not HA-pTENC124S suppressed PAK1 activation to similar levels as KID and PP2Cα.
FIGURE5.
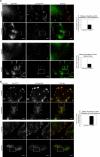
Analyses of PAK, GIT1, and paxillin loss during osmo-induced focal adhesion disassembly. A, HeLa cells were examined for effects of sorbitol treatment on adhesion-associated endogenous PAK, GIT1, and paxillin, which defines focal adhesion size. Immuno-staining conditions and data collection were identical between pairs (sorbitol treated versus untreated). Cells were plated at low density to allow for efficient spreading, and the fields of analysis were arbitrarily chosen. Regions in the cell lamella zone (away from cell body) were selected and the paxillin signals were used to define the adhesion volume using identical threshold parameters (set by the local cytosolic signal). For each paxillin-positive pixel we scored if the pixel space was also PAK-positive; colocalization percentage represents PAK-positive signal area/paxillin-positive signal area. For the sorbitol-treated cells, PAK staining after threshold assessment gives punctate random signals that contribute to “PAK-positive” pixel area, although this is likely random colocalization. For focal adhesion “size” analysis, thresholds were again set to isolate focal adhesions and paxillin-integrated fluorescence levels were quantitated within each area. This yields total paxillin signal at focal adhesions. This is repeated for each randomly selected lamella region; the mean paxillin signal per adhesion is the sum of all the integrated intensity values divided by the number of focal adhesions. Error bars on the graphs represent S.E. values. Statistics are further detailed in supplemental Table A. Sorbitol caused PAK loss from focal adhesions (over 2-fold relative to untreated) and the loss of focal adhesion integrity as measured by paxillin staining (over 2-fold relative to untreated). Regions within the images (rectangles with corresponding labels i, ii, and iii) were magnified for visualization. B, effects of sorbitol on translocation of endogenous GIT1 was examined. In contrast to PAK, GIT1 remained stably associated with focal adhesions after osmotic shock. Bars on images are equivalent to 20 μm; two independent fields were analyzed for quantitation.
FIGURE 6.
Effects of PAK inhibition on osmo-induced focal adhesion disassembly. A, HeLa cells transfected with GFP-PP2Cα (stained with anti-GFP antibody) showed protection of focal adhesion integrity compared with untransfected cells after sorbitol treatment. Focal adhesions of untransfected, untreated cells were standardized to 100% and used for normalization. After sorbitol treatment, the focal adhesions of GFP-PP2Cα expressing cells were compared with those of non-expressing cells. In the absence of sorbitol treatment, there was no significant difference between transfected and untransfected cells. Statistics are documented in supplemental Table B. B, HeLa cells transfected with GFP-KID (stained with anti-GFP antibody) similarly showed protection from focal adhesion dissolution compared with untransfected cells after sorbitol treatment. No significant difference was observed between transfected and untransfected cells without sorbitol. Bars on images are equivalent to 20 μm; two independent fields were analyzed for quantitation.
FIGURE 7.
Effects of MAPK inhibition on osmo-induced focal adhesion disassembly. A, HeLa cells were treated with DMSO control (0.1% final concentration) for 1 h before sorbitol treatment. Focal adhesions of cells not sorbitol-induced were standardized to 100% (from paxillin fluorescence levels) and used to compare with focal adhesions of sorbitol-induced cells. Statistics are detailed in supplemental Table C.B, HeLa cells were treated with 20 μ
m
SB203580 for 1 h before sorbitol treatment. Focal adhesion analysis was performed as in A. C, HeLa cells were treated with 30 μ
m
SP600125 for 1 h before sorbitol treatment. Focal adhesion analysis was performed as above.D, HeLa cells were treated with 30 μ
m
PD98059 for 1 h before sorbitol treatment. Bars on images are equivalent to 20 μm; two independent fields were analyzed for quantitation. E, COS7 cells were transfected with FLAG-ERK, with or without GST-KID. Cells were left untreated or induced with sorbitol, and FLAG-ERK immunoprecipitates were analyzed by Western blotting against phospho-ERK. Densitometry of blots was used for quantitation of osmo-induced ERK activation. The level of phospho-ERK in the first lane was standardized to 1 and used for comparison against other samples. For positive control of MEK-ERK inhibition in this assay, cells were treated with 30 μ
m
PD98059 for 1 h before sorbitol treatment, in the fifth and sixth lanes.
FIGURE 8.
Signaling through PAK in the hyperosmotic response pathway. Increased osmotic pressure signals to PI3K, resulting in the accumulation of phosphoinositides, which can be reversed by pTEN activity. This may lead to recruitment and activation of downstream effectors such as PDK1 and Rho-GEFs through their pleckstrin homology domains. PAK may be activated by Cdc42 via RhoGEF activity or directly by PDK1 phosphorylation. PAK binding to its partners PIX and GIT1, which targets the kinase to focal adhesions, is required for its activation. Osmotic pressure also activates MAPK signaling, and the MEK-ERK pathway is required for stress-induced focal adhesion disassembly and full PAK activation. It is unclear whether phosphoinositide generation links to MEK-ERK activation and whether MEK or ERK directly phosphorylates PAK in this pathway. Activated PAK acts locally at focal adhesions to promote turnover but also dissociates from them and targets to the p38 complex, which in turn regulates transcriptional response. A PAK-independent pathway is also likely to act on p38, as PAK inhibition by KID overexpression did not fully abolish p38 phosphorylation. PP2Cα may act on focal adhesion-associated PAK and on the dissociated PAK and p38 complex to down-regulate the MAPK response. Dashed lines represent intermediate or unknown steps between the signaling components.
Similar articles
- Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway.
Brown MC, West KA, Turner CE. Brown MC, et al. Mol Biol Cell. 2002 May;13(5):1550-65. doi: 10.1091/mbc.02-02-0015. Mol Biol Cell. 2002. PMID: 12006652 Free PMC article. - BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling.
Lee SH, Eom M, Lee SJ, Kim S, Park HJ, Park D. Lee SH, et al. J Biol Chem. 2001 Jul 6;276(27):25066-72. doi: 10.1074/jbc.M010892200. Epub 2001 Apr 17. J Biol Chem. 2001. PMID: 11309380 - Trihydrophobin 1 Interacts with PAK1 and Regulates ERK/MAPK Activation and Cell Migration.
Cheng C, Kong X, Wang H, Gan H, Hao Y, Zou W, Wu J, Chi Y, Yang J, Hong Y, Chen K, Gu J. Cheng C, et al. J Biol Chem. 2009 Mar 27;284(13):8786-96. doi: 10.1074/jbc.M806144200. Epub 2009 Jan 9. J Biol Chem. 2009. PMID: 19136554 Free PMC article. - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review. - p21-Activated Kinases in Thyroid Cancer.
Bautista L, Knippler CM, Ringel MD. Bautista L, et al. Endocrinology. 2020 Aug 1;161(8):bqaa105. doi: 10.1210/endocr/bqaa105. Endocrinology. 2020. PMID: 32609833 Free PMC article. Review.
Cited by
- RETRACTED: miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation.
Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. Garofalo M, et al. Cancer Cell. 2009 Dec 8;16(6):498-509. doi: 10.1016/j.ccr.2009.10.014. Cancer Cell. 2009. PMID: 19962668 Free PMC article. Retracted. - Group I and II mammalian PAKs have different modes of activation by Cdc42.
Baskaran Y, Ng YW, Selamat W, Ling FT, Manser E. Baskaran Y, et al. EMBO Rep. 2012 Jun 29;13(7):653-9. doi: 10.1038/embor.2012.75. EMBO Rep. 2012. PMID: 22653441 Free PMC article. - Increased Cell-Matrix Adhesion upon Constitutive Activation of Rho Proteins by Cytotoxic Necrotizing Factors from E. Coli and Y. Pseudotuberculosis.
May M, Kolbe T, Wang T, Schmidt G, Genth H. May M, et al. J Signal Transduct. 2012;2012:570183. doi: 10.1155/2012/570183. Epub 2012 Jul 5. J Signal Transduct. 2012. PMID: 22830013 Free PMC article. - A transcriptional cross-talk between RhoA and c-Myc inhibits the RhoA/Rock-dependent cytoskeleton.
Bustelo XR. Bustelo XR. Small GTPases. 2010 Jul;1(1):69-74. doi: 10.4161/sgtp.1.1.12986. Small GTPases. 2010. PMID: 21686122 Free PMC article. - Tissue-Specific Functions of fem-2/PP2c Phosphatase and fhod-1/formin During Caenorhabditis elegans Embryonic Morphogenesis.
Refai O, Smit RB, Votra S, Pruyne D, Mains PE. Refai O, et al. G3 (Bethesda). 2018 Jul 2;8(7):2277-2290. doi: 10.1534/g3.118.200274. G3 (Bethesda). 2018. PMID: 29720391 Free PMC article.
References
- Manser, E., Leung, T., Salihuddin, H., Zhao, Z. S., and Lim, L. (1994) Nature 367 40-46 - PubMed
- Manser, E., and Lim, L. (1999) Prog. Mol. Subcell. Biol. 22 115-133 - PubMed
- Bokoch, G. M. (2003) Annu. Rev. Biochem. 72 743-781 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous