GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia - PubMed (original) (raw)
Review
GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia
Guillermo Gonzalez-Burgos et al. Schizophr Bull. 2008 Sep.
Abstract
Synchronization of neuronal activity in the neocortex may underlie the coordination of neural representations and thus is critical for optimal cognitive function. Because cognitive deficits are the major determinant of functional outcome in schizophrenia, identifying their neural basis is important for the development of new therapeutic interventions. Here we review the data suggesting that phasic synaptic inhibition mediated by specific subtypes of cortical gamma-aminobutyric acid (GABA) neurons is essential for the production of synchronized network oscillations. We also discuss evidence indicating that GABA neurotransmission is altered in schizophrenia and propose mechanisms by which such alterations can decrease the strength of inhibitory connections in a cell-type-specific manner. We suggest that some alterations observed in the neocortex of schizophrenia subjects may be compensatory responses that partially restore inhibitory synaptic efficacy. The findings of altered neural synchrony and impaired cognitive function in schizophrenia suggest that such compensatory responses are insufficient and that interventions aimed at augmenting the efficacy of GABA neurotransmission might be of therapeutic value.
Figures
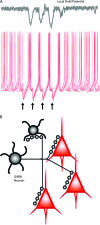
Fig. 1.
γ-aminobutyric acid (GABA) neurons are efficient at synchronizing neuronal activity in cortical networks. (A) Top, a local field potential (LFP) recorded with an extracellular electrode in the vicinity of the pyramidal neurons reflects the synchronization of pyramidal cell activity. Note the negative (downward) components of the LFP, roughly coincident with the periods of spike synchronization recorded simultaneously, as shown in the traces below. Bottom: superimposed traces of intracellular membrane potential recording, illustrating how the asynchronous firing of pyramidal neurons in response to continuous excitatory drive becomes transiently synchronized by phasic synaptic inhibition. Stimulation of an inhibitory input at the times indicated by the black arrows produces hyperpolarizing inhibitory postsynaptic potentials (IPSPs) that transiently inhibit spike firing and produce nearly synchronous spikes shortly after the IPSPs end. Data in part (A): unpublished results from G. Gonzalez-Burgos. (B) Diagram indicating that the axon of an individual GABA neuron makes multiple synaptic contacts onto multiple postsynaptic pyramidal cells and also onto other GABA neurons. The proportion of postsynaptic target cells (3 pyramidal:1 GABA) reflected in the diagram matches their relative numbers in real circuits. However, certain interneuron subtypes display marked target selectivity. For instance, chandelier neurons make synapses onto pyramidal cells but not onto other GABA neurons. In contrast, calretinin-containing interneurons synapse frequently onto other GABA neurons but rarely onto pyramidal cells (see figures 2,3 and main text).
Fig. 2.
Highly simplified view of the γ-aminobutyric acid (GABA) neuron subtypes revealed by the combined analysis of electrical, molecular, and morphological properties. Electrophysiological properties divide interneurons into 2 major clusters, fast spiking (FS) and non-fast spiking (non-FS), based on the response of the neurons’ membrane potential to direct injection of current. FS neurons form a relatively homogeneous group of interneurons that contain the calcium-binding protein parvalbumin (PV), contain no neuropeptides, and make synapses onto the perisomatic compartment of the postsynaptic pyramidal cell. FS/PV basket cells synapse onto the proximal dendrites and soma of pyramidal cells and also onto other GABA neurons. FS/PV chandelier cells synapse onto the axon initial segment of pyramidal cells but not onto other GABA neurons. The non-FS cell cluster is largely heterogeneous, containing 2 electrical subgroups, the RSNP and the BSNP neurons, the latter are also called low-threshold spiking cells (see text for additional details). An important group of non-FS cells are the Martinotti neurons, which in most cases contain the calcium-binding protein calbindin (CB) and the neuropeptides somatostatin (SST) and neuropeptide Y (NPY). Martinotti cells make synapses onto the distal dendrites of pyramidal cells. The subgroup of non-FS cells that contain the calcium-binding protein calretinin (CR) also express vasoactive intestinal peptide (VIP) and make contacts onto other GABA neurons much more frequently than onto pyramidal cells and thus are called interneuron selective. The non-FS basket cells containing cholecystokinin (CCK) do not contain PV, CB, or CR and make synapses onto the perisomatic compartment of postsynaptic pyramidal cells.
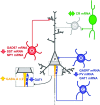
Fig. 3.
Alteration of markers of γ-aminobutyric acid (GABA) neurotransmission in the neocortex of subjects with schizophrenia. Highlighted in blue are the basket (B) and chandelier (C) neurons that contain the calcium-binding protein parvalbumin (PV), which synapse onto the soma and axon initial segment of pyramidal cells, respectively. In schizophrenia, PV-containing neurons have decreased levels of the mRNAs for PV, for the 67 kDa form of glutamic acid decarboxylase (GAD67) and for the GABA transporter 1 (GAT1). In addition, at synapses made by chandelier neurons, presynaptically there is a decrease in the concentration of GAT1 protein and postsynaptically an increase in the levels of the α2 subunits of GABA-A receptors. Schizophrenia is also associated with alterations in dendrite-targeting Martinotti neurons (M), indicated in red, in which mRNA levels are reduced for GAD67, somatostatin (SST), and neuropeptide Y (NPY). In contrast to the alterations in PV- and SST-containing neurons, GABA neurons that contain the calcium-binding protein calretinin (CR, in green), which mostly target other GABA neurons (G, in grey), do not seem to be altered in the cortex of subjects with schizophrenia. For additional details, see text.
Similar articles
- Alterations of cortical GABA neurons and network oscillations in schizophrenia.
Gonzalez-Burgos G, Hashimoto T, Lewis DA. Gonzalez-Burgos G, et al. Curr Psychiatry Rep. 2010 Aug;12(4):335-44. doi: 10.1007/s11920-010-0124-8. Curr Psychiatry Rep. 2010. PMID: 20556669 Free PMC article. Review. - GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia.
Gonzalez-Burgos G, Fish KN, Lewis DA. Gonzalez-Burgos G, et al. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. Epub 2011 Sep 5. Neural Plast. 2011. PMID: 21904685 Free PMC article. Review. - Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons.
Lewis DA, Hashimoto T. Lewis DA, et al. Int Rev Neurobiol. 2007;78:109-31. doi: 10.1016/S0074-7742(06)78004-7. Int Rev Neurobiol. 2007. PMID: 17349859 Review. - Altered Gradients of Glutamate and Gamma-Aminobutyric Acid Transcripts in the Cortical Visuospatial Working Memory Network in Schizophrenia.
Hoftman GD, Dienel SJ, Bazmi HH, Zhang Y, Chen K, Lewis DA. Hoftman GD, et al. Biol Psychiatry. 2018 Apr 15;83(8):670-679. doi: 10.1016/j.biopsych.2017.11.029. Epub 2017 Dec 7. Biol Psychiatry. 2018. PMID: 29357982 Free PMC article. - [Schizophrenia and cortical GABA neurotransmission].
Hashimoto T, Matsubara T, Lewis DA. Hashimoto T, et al. Seishin Shinkeigaku Zasshi. 2010;112(5):439-52. Seishin Shinkeigaku Zasshi. 2010. PMID: 20560363 Review. Japanese.
Cited by
- Abnormal phase entrainment of low- and high-gamma-band auditory steady-state responses in schizophrenia.
Nakanishi S, Tamura S, Hirano S, Takahashi J, Kitajima K, Takai Y, Mitsudo T, Togao O, Nakao T, Onitsuka T, Hirano Y. Nakanishi S, et al. Front Neurosci. 2023 Oct 24;17:1277733. doi: 10.3389/fnins.2023.1277733. eCollection 2023. Front Neurosci. 2023. PMID: 37942136 Free PMC article. - The Role of Parvalbumin-positive Interneurons in Auditory Steady-State Response Deficits in Schizophrenia.
Metzner C, Zurowski B, Steuber V. Metzner C, et al. Sci Rep. 2019 Dec 6;9(1):18525. doi: 10.1038/s41598-019-53682-5. Sci Rep. 2019. PMID: 31811155 Free PMC article. - Paranormal believers show reduced resting EEG beta band oscillations and inhibitory control than skeptics.
Narmashiri A, Hatami J, Khosrowabadi R, Sohrabi A. Narmashiri A, et al. Sci Rep. 2023 Feb 24;13(1):3258. doi: 10.1038/s41598-023-30457-7. Sci Rep. 2023. PMID: 36828909 Free PMC article. - Deletion of Fmr1 from Forebrain Excitatory Neurons Triggers Abnormal Cellular, EEG, and Behavioral Phenotypes in the Auditory Cortex of a Mouse Model of Fragile X Syndrome.
Lovelace JW, Rais M, Palacios AR, Shuai XS, Bishay S, Popa O, Pirbhoy PS, Binder DK, Nelson DL, Ethell IM, Razak KA. Lovelace JW, et al. Cereb Cortex. 2020 Mar 14;30(3):969-988. doi: 10.1093/cercor/bhz141. Cereb Cortex. 2020. PMID: 31364704 Free PMC article. - Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia.
Lewis DA. Lewis DA. Curr Opin Neurobiol. 2014 Jun;26:22-6. doi: 10.1016/j.conb.2013.11.003. Epub 2013 Nov 30. Curr Opin Neurobiol. 2014. PMID: 24650500 Free PMC article. Review.
References
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. - PubMed
- Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. - PubMed
- Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical