Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP - PubMed (original) (raw)
Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP
Madelyn S Hanson et al. Am J Physiol Heart Circ Physiol. 2008 Aug.
Abstract
Increases in the second messenger cAMP are associated with receptor-mediated ATP release from erythrocytes. In other signaling pathways, cAMP-specific phosphodiesterases (PDEs) hydrolyze this second messenger and thereby limit its biological actions. Although rabbit and human erythrocytes possess adenylyl cyclase and synthesize cAMP, their PDE activity is poorly characterized. It was reported previously that the prostacyclin analog iloprost stimulated receptor-mediated increases in cAMP in rabbit and human erythrocytes. However, the PDEs that hydrolyze erythrocyte cAMP synthesized in response to iloprost were not identified. PDE3 inhibitors were reported to augment increases in cAMP stimulated by prostacyclin analogs in platelets and pulmonary artery smooth muscle cells. Additionally, PDE3 activity was identified in embryonic avian erythrocytes, but the presence of this PDE in mammalian erythrocytes has not been investigated. Here, using Western blot analysis, we determined that PDE3B is a component of rabbit and human erythrocyte membranes. In addition, we report that the preincubation of rabbit and human erythrocytes with the PDE3 inhibitors milrinone and cilostazol potentiates iloprost-induced increases in cAMP. In addition, cilostamide, the parent compound of cilostazol, potentiated iloprost-induced increases in cAMP in human erythrocytes. These findings demonstrate that PDE3B is present in rabbit and human erythrocytes and are consistent with the hypothesis that PDE3 activity regulates cAMP levels associated with a signaling pathway activated by iloprost in these cells.
Figures
Fig. 1.
Identification of phosphodiesterase 3B (PDE 3B) in rabbit and human erythrocyte membranes. A: human erythrocyte membranes and purified human PDE3B were probed with a polyclonal antibody generated against the NH2 terminus of human PDE3B. B: rabbit and human erythrocyte membranes were probed with either a polyclonal antibody directed against the COOH terminus of human PDE3B (rabbit erythrocyte membranes, representative of 6 studies) or a polyclonal antibody generated against the NH2 terminus of human PDE3B (human erythrocyte membranes, representative of 14 studies).
Fig. 2.
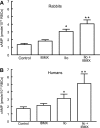
Effect of 3-isobutyl-1-methylxanthine (IBMX; 10 μM) on iloprost (Ilo)-induced levels of cAMP in rabbit and human erythrocytes. A: washed erythrocytes from rabbits (n = 5) were incubated with Ilo (1 μM) in the presence of 10 μM IBMX or its vehicle [_N,N_-dimethylformamide (DMF)]. B: washed erythrocytes from humans (n = 8) were incubated with Ilo (1 μM) in the presence of 10 μM IBMX or its vehicle (DMF). In both A and B, cells were incubated with IBMX or its vehicle (DMF) for 30 min, and then cAMP increases were stimulated by the addition of Ilo for 15 min. Values are means ± SE. P < 0.05; *different from control and inhibitor alone; **greater than all other values. RBCs, red blood cells.
Fig. 3.
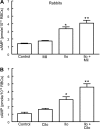
Effect of inhibitors of PDE3 on Ilo-induced increases in cAMP in rabbit erythrocytes. A: washed erythrocytes from rabbits (n = 6) were incubated with Ilo (1 μM) in the presence of 20 μM milrinone (Mil) or its vehicle (DMF). B: washed erythrocytes from rabbits (n = 6) were incubated with Ilo (1 μM) in the presence of 10 μM cilostazol (Cilo) or its vehicle (DMF). Cells were incubated with Mil, Cilo, or DMF for 30 min, and then cAMP increases were stimulated by the addition of Ilo for 15 min. Values are means ± SE. P < 0.05; *different from control and inhibitor alone; **greater than all other values.
Fig. 4.
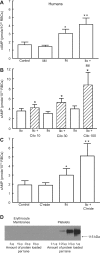
Effect of PDE3 inhibitors on Ilo-induced levels of cAMP in human erythrocytes. A: washed erythrocytes from humans (n = 6) were incubated with Ilo (1 μM) in the presence of 20 μM Mil or its vehicle (DMF). B: washed erythrocytes from humans were incubated with Ilo (1 μM) in the presence of 10 μM (n = 12), 30 μM (n = 9), or 100 μM (n = 6) Cilo or its vehicle (DMF). C: washed erythrocytes from humans (n = 4) were incubated with Ilo (1 μM) in the presence of 30 μM cilostamide (C'mide) or its vehicle (DMF). Cells were incubated with Mil, Cilo, C'mide, or DMF for 30 min, and then cAMP increases were stimulated by the addition of Ilo for 15 min. Values are means ± SE. P < 0.05; *different from control and inhibitor alone (A and C) or corresponding Ilo (B); **greater than all other values. D: human erythrocyte membranes and human platelet membranes (representative of 7 studies) were probed with a monoclonal antibody generated against integrin αIIb (CD41).
Similar articles
- Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans.
Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Adderley SP, et al. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1617-24. doi: 10.1152/ajpheart.01226.2008. Epub 2009 Feb 27. Am J Physiol Heart Circ Physiol. 2009. PMID: 19252089 Free PMC article. - Comparison of the effects of cilostazol and milrinone on cAMP-PDE activity, intracellular cAMP and calcium in the heart.
Shakur Y, Fong M, Hensley J, Cone J, Movsesian MA, Kambayashi J, Yoshitake M, Liu Y. Shakur Y, et al. Cardiovasc Drugs Ther. 2002 Sep;16(5):417-27. doi: 10.1023/a:1022186402442. Cardiovasc Drugs Ther. 2002. PMID: 12652111 - Protein kinases A and C regulate receptor-mediated increases in cAMP in rabbit erythrocytes.
Adderley SP, Sridharan M, Bowles EA, Stephenson AH, Ellsworth ML, Sprague RS. Adderley SP, et al. Am J Physiol Heart Circ Physiol. 2010 Feb;298(2):H587-93. doi: 10.1152/ajpheart.00975.2009. Epub 2009 Dec 11. Am J Physiol Heart Circ Physiol. 2010. PMID: 20008267 Free PMC article. - Regulation of cAMP by phosphodiesterases in erythrocytes.
Adderley SP, Sprague RS, Stephenson AH, Hanson MS. Adderley SP, et al. Pharmacol Rep. 2010 May-Jun;62(3):475-82. doi: 10.1016/s1734-1140(10)70303-0. Pharmacol Rep. 2010. PMID: 20631411 Free PMC article. Review. - Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake.
Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Liu Y, et al. Cardiovasc Drug Rev. 2001 Winter;19(4):369-86. doi: 10.1111/j.1527-3466.2001.tb00076.x. Cardiovasc Drug Rev. 2001. PMID: 11830753 Review.
Cited by
- Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP.
Ellsworth ML, Sprague RS. Ellsworth ML, et al. J Physiol. 2012 Oct 15;590(20):4985-91. doi: 10.1113/jphysiol.2012.233106. Epub 2012 May 14. J Physiol. 2012. PMID: 22586223 Free PMC article. Review. - Erythrocyte-derived ATP and perfusion distribution: role of intracellular and intercellular communication.
Sprague RS, Ellsworth ML. Sprague RS, et al. Microcirculation. 2012 Jul;19(5):430-9. doi: 10.1111/j.1549-8719.2011.00158.x. Microcirculation. 2012. PMID: 22775760 Free PMC article. Review. - Erythrocytes Induce Vascular Dysfunction in COVID-19.
Mahdi A, Collado A, Tengbom J, Jiao T, Wodaje T, Johansson N, Farnebo F, Färnert A, Yang J, Lundberg JO, Zhou Z, Pernow J. Mahdi A, et al. JACC Basic Transl Sci. 2022 Mar;7(3):193-204. doi: 10.1016/j.jacbts.2021.12.003. Epub 2022 Feb 16. JACC Basic Transl Sci. 2022. PMID: 35194565 Free PMC article. - Identification of cytosolic phosphodiesterases in the erythrocyte: a possible role for PDE5.
Adderley SP, Thuet KM, Sridharan M, Bowles EA, Stephenson AH, Ellsworth ML, Sprague RS. Adderley SP, et al. Med Sci Monit. 2011 May;17(5):CR241-7. doi: 10.12659/msm.881763. Med Sci Monit. 2011. PMID: 21525805 Free PMC article. - A novel blood-feeding detoxification pathway in Nippostrongylus brasiliensis L3 reveals a potential checkpoint for arresting hookworm development.
Bouchery T, Filbey K, Shepherd A, Chandler J, Patel D, Schmidt A, Camberis M, Peignier A, Smith AAT, Johnston K, Painter G, Pearson M, Giacomin P, Loukas A, Bottazzi ME, Hotez P, LeGros G. Bouchery T, et al. PLoS Pathog. 2018 Mar 22;14(3):e1006931. doi: 10.1371/journal.ppat.1006931. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29566094 Free PMC article.
References
- Babu CR, Azhar S, Krishna Murti CR. Loss of epinephrine stimulated synthesis of cyclic adenosine 3′:5′ monophosphate during maturation of rabbit and human reticulocytes. Med Biol 53: 148–155, 1975. - PubMed
- Baumann R, Blass C, Gotz R, Dragon S. Ontogeny of catecholamine and adenosine receptor-mediated cAMP signaling of embryonic red blood cells: role of cGMP-inhibited phosphodiesterase 3 and hemoglobin. Blood 94: 4314–4320, 1999. - PubMed
- Beaumont C, Piau JP, Fischer S, Delaunay J, Schapira G. Stimulation of erythroid cells adenylate cyclase by soluble factors. Biochem Biophys Res Commun 91: 1250–1257, 1979. - PubMed
- Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem 272: 6823–6826, 1997. - PubMed
- Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases