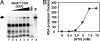Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import - PubMed (original) (raw)
Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import
Mary Anne T Rubio et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Mitochondrial genomes generally encode a minimal set of tRNAs necessary for protein synthesis. However, a number of eukaryotes import tRNAs from the cytoplasm into their mitochondria. For instance, Saccharomyces cerevisiae imports cytoplasmic tRNA(Gln) into the mitochondrion without any added protein factors. Here, we examine the existence of a similar active tRNA import system in mammalian mitochondria. We have used subcellular RNA fractions from rat liver and human cells to perform RT-PCR with oligonucleotide primers specific for nucleus-encoded tRNA(CUG)(Gln) and tRNA(UUG)(Gln) species, and we show that these tRNAs are present in rat and human mitochondria in vivo. Import of in vitro transcribed tRNAs, but not of heterologous RNAs, into isolated mitochondria also demonstrates that this process is tRNA-specific and does not require the addition of cytosolic factors. Although this in vitro system requires ATP, it is resistant to inhibitors of the mitochondrial electrochemical gradient, a key component of protein import. tRNA(Gln) import into mammalian mitochondria proceeds by a mechanism distinct from protein import. We also show that both tRNA(Gln) species and a bacterial pre-tRNA(Asp) can be imported in vitro into mitochondria isolated from myoclonic epilepsy with ragged-red fiber cells if provided with sufficient ATP (2 mM). This work suggests that tRNA import is more widespread than previously thought and may be a universal trait of mitochondria. Mutations in mitochondrial tRNA genes have been associated with human disease; the tRNA import system described here could possibly be exploited for the manipulation of defective mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
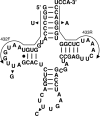
Fig. 1.
Secondary structure of the nucleus-encoded tRNAGln from rat. Arrowheads indicate the single-nucleotide differences between the six different isoacceptors found in the genome. Arrows indicate the position of the two oligonucleotide primers used for RT-PCR amplification (432F and 433R). These two primers can only discriminate four of the possible six isoacceptors. The same primers were used for the analysis of nucleus-encoded tRNAGln from humans.
Fig. 2.
Nucleus-encoded tRNAGln is imported into rat and human mitochondria. (A) RT-PCR with primers specific for the nucleus-encoded tRNAGln (ntRNAGln) isoacceptors and total RNA isolated from rat (Left) or human (Right) mitochondria. These primers are unable to amplify the endogenous mitochondria-encoded tRNAUUGGln (mttRNAUUGGln) and can only discriminate between four of the possible six nucleus-encoded isoacceptors. (B) Similar RT-PCR as in A but with extramitochondrial RNA, which includes a mixture of cytoplasmic as well as nuclear RNA generated from the same preparation as that used to isolate the mitochondria in A. (C) RT-PCR with U6-specific oligonucleotides (extramitochondrial marker) with the same RNA fractions as in A and B. RT+ and RT− refer to cDNA synthesis reactions performed in the presence or absence of reverse transcriptase, respectively. No template is a mock reaction in which no cDNA was added, also serving as a negative control. Mito, RNA isolated from purified mitochondria. M, a 100-bp DNA ladder used as size markers during electrophoresis.
Fig. 3.
Distribution of imported tRNAGln species in rat mitochondrial RNA fractions. (Left) Table summarizing the total distribution of each isoacceptor in the various fractions. Similar numbers were obtained for human mitochondria (data not shown). (Right) DNA sequencing traces representative of the different products obtained from the RT-PCR experiments in (Fig. 2). The single-nucleotide differences that can be discerned with the primers used are underlined. Also underlined are the anticodon sequences.
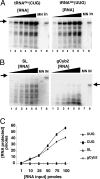
Fig. 4.
Nucleus-encoded tRNAGln is efficiently and specifically imported into isolated rat liver mitochondria in vitro. (A) Increasing concentrations of radioactively labeled ntRNACUGGln (Left) or ntRNAUUGGln transcripts (Right) were incubated with constant concentrations of Percoll-purified mitochondria as described in Materials and Methods. (B) Reactions similar to those in A but with two heterologous RNAs as specificity controls for in vitro import, where SL and gCyb2 refer to spliced leader and gRNA transcripts from trypanosomatids. These RNAs do not exist in mammalian cells. (C) A plot of the results from A and B where pmoles of input RNA protected are plotted vs. the input. MN (lanes 7), control reactions treated with micrococcal nuclease in the absence of mitochondria. These reactions serve as a control for MN digestion. IN, input lane of the original tRNA used in the import experiments and serving as a quantitation standard. The black triangle denotes increasing concentrations of a given reagent used in the assay (at 1, 10, 25, 50, 80, and 100 pmol of input, lanes 1–6 in A and B). The arrow marks the position of the full-length RNA after gel electrophoresis as described in Materials and Methods.
Fig. 5.
Import of tRNA requires ATP. (A) Constant concentrations of radioactively labeled tRNACUGGln or tRNAUUGGln (data not shown) were incubated in the absence (lane 1) or in the presence of increasing ATP (at 0.5, 1, 2, 5, 10, and 15 mM final concentration, lanes 2–8) yielding identical results. (B) Plot of the protected tRNA fraction following import vs. ATP concentration. MN and IN (lanes 7 and 8) are as described in the legend to Fig. 4.
Fig. 6.
tRNA import occurs by a mechanism distinct from protein import. (A) The import of tRNACUGGln was examined in purified mitochondria pretreated with digitonin (at 0.01, 0.1, 0.5, 1, and 5% final concentration; lanes 1–8, respectively) and compared with a standard import reaction (Std, lane 9) without pretreatment. In addition, mitochondria were pretreated with proteinase K (PK, lanes 13 and 14) or mildly treated with SDS (lanes 15 and 16). PK (no mito), incubation of the labeled transcript in the presence of an amount of proteinase K equivalent to that used in the assay in lane 13 but in the absence of mitochondria, demonstrating that the lack of protection in lanes 13 and 14 cannot be ascribed to degradation of the tRNA by proteinase K. (B) Mitochondria were also treated with increasing concentrations of oligomycin (1.3, 3.2, 31.7, 63.3, 95, and 126 nM; lanes 3–8, respectively) to inhibit ATPase activity in the presence of KCN. Import of tRNA under these conditions was compared with a standard import reaction (Std, lane 1) and a standard reaction with KCN alone (lane 2). In addition, tRNAs were also imported into mitochondria treated with ethanol (EtOH) (lane 10) or KCN + ethanol (EtOH+KCN, lane 9) alone serving as a control for the various cofactors and solvents. (C) Mitochondria were also treated with increasing concentrations of the potassium ionophore valinomycin (1, 5, 10, and 20 μM; lanes 1–4, respectively) in the presence of KCl (70 mM final concentration). The highest level of valinomycin used represents 100× the concentration required to collapse the membrane potential (data not shown). Val+KCl (lane 5), KCl only (lane 6), and EtOH only (lane 7) refer to import reactions in the presence of these various components used as cofactors or solvents in the valinomycin experiment. Incubation of tRNA alone with any of these components does not inhibit MN cleavage (data not shown). Similar reactions were performed in reactions where valinomycin was replaced by nigericin (Nig) (1, 5, 10, and 20 μM; lanes 11–14), a potent inhibitor of the pH gradient. nig (−) KCL (lanes 15 and 16) refers to an import reaction in the presence of nigericin but in the absence of KCl. Control reactions without mitochondria and in the presence of nigericin showed that nigericin does not inhibit MN cleavage (data not shown). (D) tRNA import assays with mitochondria treated with the uncoupler CCCP at concentrations well above what is required to destroy the electrochemical gradient (0.1, 0.25, 0.5, 0.75, 1.0, 1.5, and 2 mM; lanes 2–8, respectively). Because CCCP is dissolved in methanol, control import reactions in the presence of methanol are also shown (lanes 9 and 10). Throughout the figure, arrows denote the full-length tRNA substrate, and the black triangle represents increasing or decreasing concentrations of a given chemical. MN and IN, control reactions for nuclease cleavage and as a quantitation standard, respectively.
Fig. 7.
Addition of ATP rescues the ability of diseased mitochondria to import tRNAGln. (A) Radioactively labeled tRNACUGGln was incubated with mitochondria isolated from wild-type (WT) human cells, and their import behavior was compared with that isolated from cybrid cells originally generated from patients with a mitochondrial defect that causes MERRF. Mitochondria were Percoll-purified as described in Materials and Methods. Import experiments were performed in the absence (−) or presence (+) of ATP. MN, control reactions treated with micrococcal nuclease in the absence of mitochondria. These reactions serve as a control for MN digestion. IN, input lane of the original tRNA used in the import experiments and serving as a quantitation standard. Black arrows mark the position of the full-length RNA, and the gray arrow marks the position of the mature tRNA processed in the organelle following import. All import reactions were analyzed by gel electrophoresis as described in Materials and Methods. Lanes 1–4 show import reactions using a transcript corresponding to the nucleus-encoded human tRNACUGGln. Lanes 5–10 show reactions similar to those in lanes 1–4, except that pre-tRNAAsp from Bacillus was used in the import reactions to demonstrate transport of the tRNA into the matrix. (B) A reaction similar to that in lane 6 of A was analyzed by a primer extension assay to map the 5′ end of the products generated after import as described in ref. . Lanes 1 and 2 are negative control reactions performed in the absence of an RNA template or the absence of reverse transcriptase. Lane 3, in vitro cleavage reaction with Escherichia coli RNase P (140 pmol) incubated with the precursor tRNAAsp (40 pmol) as described in ref. , which yields a mature tRNA and serves as a size marker. Lane 4, result of an RNA import reaction with MERRF mitochondria and unlabeled tRNAAsp. Lane 5, full-length pre-tRNA serving as a control and size marker. G, A, T, and C denote an unrelated sequencing ladder used as size markers for the reaction. The dashed arrow corresponds to the 5′ end of pre-tRNAAsp after treatment with RNase P. The solid arrow marks the position of the pre-tRNAAsp, and the bracket marks the position of the cleavage product generated by the mitochondrial matrix localized nuclease activity found in mammalian mitochondria and described in ref. . This activity has been shown to digest bacterial pre-tRNAs at 2–4 nucleotides from the authentic 5′ end of the mature product (31).
Similar articles
- Mitochondrial import of only one of three nuclear-encoded glutamine tRNAs in Tetrahymena thermophila.
Rusconi CP, Cech TR. Rusconi CP, et al. EMBO J. 1996 Jul 1;15(13):3286-95. EMBO J. 1996. PMID: 8670829 Free PMC article. - The anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena.
Rusconi CP, Cech TR. Rusconi CP, et al. Genes Dev. 1996 Nov 15;10(22):2870-80. doi: 10.1101/gad.10.22.2870. Genes Dev. 1996. PMID: 8918888 - Selective import of nuclear-encoded tRNAs into mitochondria of the protozoan Leishmania tarentolae.
Lye LF, Chen DH, Suyama Y. Lye LF, et al. Mol Biochem Parasitol. 1993 Apr;58(2):233-45. doi: 10.1016/0166-6851(93)90045-y. Mol Biochem Parasitol. 1993. PMID: 8479448 - Transfer RNA travels from the cytoplasm to organelles.
Rubio MA, Hopper AK. Rubio MA, et al. Wiley Interdiscip Rev RNA. 2011 Nov-Dec;2(6):802-17. doi: 10.1002/wrna.93. Epub 2011 Jul 11. Wiley Interdiscip Rev RNA. 2011. PMID: 21976284 Free PMC article. Review. - Mitochondrial tRNA import and its consequences for mitochondrial translation.
Schneider A. Schneider A. Annu Rev Biochem. 2011;80:1033-53. doi: 10.1146/annurev-biochem-060109-092838. Annu Rev Biochem. 2011. PMID: 21417719 Review.
Cited by
- Co-evolution of mitochondrial tRNA import and codon usage determines translational efficiency in the green alga Chlamydomonas.
Salinas T, Duby F, Larosa V, Coosemans N, Bonnefoy N, Motte P, Maréchal-Drouard L, Remacle C. Salinas T, et al. PLoS Genet. 2012 Sep;8(9):e1002946. doi: 10.1371/journal.pgen.1002946. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028354 Free PMC article. - Mitochondria, Telomeres and Telomerase Subunits.
Zheng Q, Huang J, Wang G. Zheng Q, et al. Front Cell Dev Biol. 2019 Nov 6;7:274. doi: 10.3389/fcell.2019.00274. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31781563 Free PMC article. Review. - Biogenesis of glutaminyl-mt tRNAGln in human mitochondria.
Nagao A, Suzuki T, Katoh T, Sakaguchi Y, Suzuki T. Nagao A, et al. Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16209-14. doi: 10.1073/pnas.0907602106. Epub 2009 Sep 9. Proc Natl Acad Sci U S A. 2009. PMID: 19805282 Free PMC article. - Extension of Mitogenome Enrichment Based on Single Long-Range PCR: mtDNAs and Putative Mitochondrial-Derived Peptides of Five Rodent Hibernators.
Emser SV, Schaschl H, Millesi E, Steinborn R. Emser SV, et al. Front Genet. 2021 Dec 13;12:685806. doi: 10.3389/fgene.2021.685806. eCollection 2021. Front Genet. 2021. PMID: 35027919 Free PMC article. - The Roles of CircRNAs in Mitochondria.
Liu D, Zhou X, He Y, Zhao J. Liu D, et al. J Cancer. 2024 Mar 17;15(9):2759-2769. doi: 10.7150/jca.92111. eCollection 2024. J Cancer. 2024. PMID: 38577612 Free PMC article. Review.
References
- Rorbach J, Soleimanpour-Lichaei R, Lightowlers RN, Chrzanowska-Lightowlers ZM. How do mammalian mitochondria synthesize proteins? Biochem Soc Trans. 2007;35:1290–1291. - PubMed
- Schneider A. Does the evolutionary history of aminoacyl-tRNA synthetases explain the loss of mitochondrial tRNA genes? Trends Genet. 2001;17:557–559. - PubMed
- Schneider A, Marechal-Drouard L. Mitochondrial tRNA import: Are there distinct mechanisms? Trends Cell Biol. 2000;10:509–513. - PubMed
- Suyama Y. The origins of mitochondrial ribonucleic acids in Tetrahymena pyriformis. Biochemistry. 1967;6:2829–2839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources