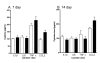Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury - PubMed (original) (raw)
Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury
Eric Lloyd et al. J Neuroinflammation. 2008.
Abstract
Background: Traumatic brain injury (TBI) with its associated morbidity is a major area of unmet medical need that lacks effective therapies. TBI initiates a neuroinflammatory cascade characterized by activation of astrocytes and microglia, and increased production of immune mediators including proinflammatory cytokines and chemokines. This inflammatory response contributes both to the acute pathologic processes following TBI including cerebral edema, in addition to longer-term neuronal damage and cognitive impairment. However, activated glia also play a neuroprotective and reparative role in recovery from injury. Thus, potential therapeutic strategies targeting the neuroinflammatory cascade must use careful dosing considerations, such as amount of drug and timing of administration post injury, in order not to interfere with the reparative contribution of activated glia.
Methods: We tested the hypothesis that attenuation of the acute increase in proinflammatory cytokines and chemokines following TBI would decrease neurologic injury and improve functional neurologic outcome. We used the small molecule experimental therapeutic, Minozac (Mzc), to suppress TBI-induced up-regulation of glial activation and proinflammatory cytokines back towards basal levels. Mzc was administered in a clinically relevant time window post-injury in a murine closed-skull, cortical impact model of TBI. Mzc effects on the acute increase in brain cytokine and chemokine levels were measured as well as the effect on neuronal injury and neurobehavioral function.
Results: Administration of Mzc (5 mg/kg) at 3 h and 9 h post-TBI attenuates the acute increase in proinflammatory cytokine and chemokine levels, reduces astrocyte activation, and the longer term neurologic injury, and neurobehavioral deficits measured by Y maze performance over a 28-day recovery period. Mzc-treated animals also have no significant increase in brain water content (edema), a major cause of the neurologic morbidity associated with TBI.
Conclusion: These results support the hypothesis that proinflammatory cytokines contribute to a glial activation cycle that produces neuronal dysfunction or injury following TBI. The improvement in long-term functional neurologic outcome following suppression of cytokine upregulation in a clinically relevant therapeutic window indicates that selective targeting of neuroinflammation may lead to novel therapies for the major neurologic morbidities resulting from head injury, and indicates the potential of Mzc as a future therapeutic for TBI.
Figures
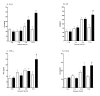
Figure 1
Time course of acute changes in proinflammatory cytokines following TBI. Levels of the cytokines IL-1β (A), IL6 (B), TNFα (C) and the chemokine CCL2 (D) in pooled hippocampus and cortex extracts following sham procedure (open bars) or closed head TBI (filled bars) were measured by ELISA. Animals were sacrificed at 0-, 1-, 4-, and 12-hr recovery. Data are expressed as mean ± S.E.M of n = 6–8 animals per group. Significantly different from sham: *P < 0.05 vs Sham control; **P < 0.01 vs Sham; #P < 0.001 vs Sham by ANOVA.
Figure 2
Time course of long-term changes in proinflammatory cytokines following TBI. Levels of the cytokines IL-1β, IL6, TNFα, and the chemokine CCL2 in pooled hippocampus and cortex extracts following sham procedure (open bars) or closed head TBI (filled bars) were measured by ELISA. Animals were sacrificed at 7 (A) and 14 (B) day recovery. Data are expressed as mean ± S.E.M of n = 6–8 animals per group. There were no significant differences between groups at either time point.
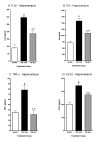
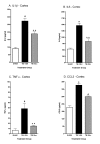
Figure 3
Minozac suppresses proinflammatory cytokine upregulation in hippocampus following TBI. Mice were subjected to TBI or sham procedure. At 3 hr and 9 hr following TBI, mice were injected with Mzc (5 mg/kg/dose) or saline diluent (VEH). Mice were sacrificed at 12 hr post-injury, and levels of the proinflammatory cytokines IL-1β (A), IL6 (B), TNFα (C) and the chemokine CCL2 (D) in hippocampal extracts were measured by ELISA. Mzc treatment resulted in significant attenuation of the increase in cytokines measured in the TBI group treated with saline vehicle. Cytokine levels in the Mzc-treated TBI mice were not significantly different from the sham controls. Data are expressed as mean ± S.E.M of n = 5–7 animals per group. #P < 0.001 vs Sham control; **P < 0.01 vs TBI-VEH by ANOVA.
Figure 4
Minozac suppresses proinflammatory cytokine upregulation in cortex following TBI. Mice were subjected to TBI or sham procedure. At 3 hr and 9 hr following TBI, mice were injected with Mzc (5 mg/kg/dose) or saline diluent (VEH). Mice were sacrificed at 12 hr post-injury, and levels of the proinflammatory cytokines IL-1β (A), IL6 (B), TNFα (C) and the chemokine CCL2 (D) in cortical extracts were measured by ELISA. Mzc treatment resulted in significant attenuation of the increase in cytokines measured in the TBI group treated with saline vehicle. Cytokine levels in the Mzc-treated TBI mice were not significantly different from the sham controls. Data are expressed as mean ± S.E.M of n = 5–7 animals per group. #P < 0.001 vs Sham control; **P < 0.01 vs TBI-VEH by ANOVA.
Figure 5
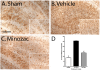
Quantification of S100B immunoreactive cells after 28 day recovery following TBI. Mice were subjected to TBI or sham procedure. At 3 hr and 9 hr following TBI, mice were injected with Mzc (5 mg/kg/dose) or saline diluent (VEH). After 28 day recovery, S100B immunoreactive cells in the hippocampus were quantified in sham controls (A), TBI treated with saline vehicle (B) and TBI treated with Mzc (C). Representative sections show an increase in the injured animals (B) which was prevented by treatment with Mzc (C). Insets (A-C) show high power image of hippocampal neuronal layer. Quantification of the digitized images (D) shows a reduction in astrocyte activation in the Mzc-treated group. Data are expressed as mean ± S.E.M of n = 5–7 animals per group. #P < 0.001 vs TBI-Veh by ANOVA. Bar = 100 μm.
Figure 6
Minozac attenuates neuronal injury after 28 day recovery following TBI. Mice were subjected to TBI or sham procedure. At 3 hr and 9 hr following TBI, mice were injected with Mzc (5 mg/kg/dose) or saline diluent (VEH). After 28-day recovery, neuronal injury was quantified in each region of the hippocampus based on the morphologic appearance of the NeuN-labeled neurons and a score (1 = normal; 2= moderate injury; 3= severe injury) assigned for each region. Representative sections are shown for sham controls (A), TBI treated with saline vehicle (B) and TBI treated with Mzc (C). Neurons in injured animals treated with saline were shrunken and dystrophic (B) compared to both the injured animals treated with Mzc (C) and sham controls (A). (D) Quantification of the regional injury scores between groups. Data are expressed as median injury score ± IQR of n = 5–7 animals per group. *P < 0.05 vs TBI-VEH by ANOVA. Bar = 100 μm.
Figure 7
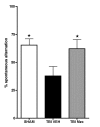
Minozac attenuates hippocampal-dependent Y-maze behavioral impairment following TBI. Mice were subjected to TBI or sham procedure. At 3 hr and 9 hr following TBI, mice were injected with Mzc (5 mg/kg/dose) or saline diluent (VEH) via intraperitoneal injection. On days 7 through 28 of recovery, hippocampal function was assessed by alternation in the Y-maze. Treatment with Mzc prevented neurobehavioral impairment resulting from TBI. Data are expressed as mean ± S.E.M of n = 5–7 animals per group. *P < 0.05 vs TBI-VEH by ANOVA.
Figure 8
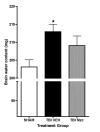
Minozac treated animals have attenuated increase in brain water content following TBI. Mice were subjected to TBI or sham procedure. At 3 hr and 9 hr following TBI, mice were injected with Mzc (5 mg/kg/dose) or saline diluent (VEH) via intraperitoneal injection. Mice were sacrificed 24 hr post-injury, and brain water content was measured in coronal sections by wet dry methods. Water content increased significantly following TBI in mice treated with saline but not when treated with Mzc. Data are expressed as mean ± S.E.M of n = 9–13 animals per group. *P < 0.05 vs sham control by ANOVA.
Similar articles
- Minozac treatment prevents increased seizure susceptibility in a mouse "two-hit" model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures.
Chrzaszcz M, Venkatesan C, Dragisic T, Watterson DM, Wainwright MS. Chrzaszcz M, et al. J Neurotrauma. 2010 Jul;27(7):1283-95. doi: 10.1089/neu.2009.1227. J Neurotrauma. 2010. PMID: 20486807 Free PMC article. - Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic.
Bachstetter AD, Webster SJ, Goulding DS, Morton JE, Watterson DM, Van Eldik LJ. Bachstetter AD, et al. J Neuroinflammation. 2015 Apr 10;12:69. doi: 10.1186/s12974-015-0289-5. J Neuroinflammation. 2015. PMID: 25886256 Free PMC article. - Interferon-β Plays a Detrimental Role in Experimental Traumatic Brain Injury by Enhancing Neuroinflammation That Drives Chronic Neurodegeneration.
Barrett JP, Henry RJ, Shirey KA, Doran SJ, Makarevich OD, Ritzel RM, Meadows VA, Vogel SN, Faden AI, Stoica BA, Loane DJ. Barrett JP, et al. J Neurosci. 2020 Mar 11;40(11):2357-2370. doi: 10.1523/JNEUROSCI.2516-19.2020. Epub 2020 Feb 6. J Neurosci. 2020. PMID: 32029532 Free PMC article. - Genetic Influences in Traumatic Brain Injury.
Bennett ER, Reuter-Rice K, Laskowitz DT. Bennett ER, et al. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 9. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 9. PMID: 26583176 Free Books & Documents. Review. - Models of Posttraumatic Brain Injury Neurorehabilitation.
Failla MD, Wagner AK. Failla MD, et al. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 35. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 35. PMID: 26269917 Free Books & Documents. Review.
Cited by
- Traumatic brain injury induces macrophage subsets in the brain.
Hsieh CL, Kim CC, Ryba BE, Niemi EC, Bando JK, Locksley RM, Liu J, Nakamura MC, Seaman WE. Hsieh CL, et al. Eur J Immunol. 2013 Aug;43(8):2010-22. doi: 10.1002/eji.201243084. Epub 2013 Jun 5. Eur J Immunol. 2013. PMID: 23630120 Free PMC article. - Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-κB signaling after experimental traumatic brain injury.
Chen CC, Hung TH, Wang YH, Lin CW, Wang PY, Lee CY, Chen SF. Chen CC, et al. PLoS One. 2012;7(1):e30294. doi: 10.1371/journal.pone.0030294. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272328 Free PMC article. - Sex-stratified RNA-seq analysis reveals traumatic brain injury-induced transcriptional changes in the female hippocampus conducive to dementia.
Fiorini MR, Dilliott AA, Farhan SMK. Fiorini MR, et al. Front Neurol. 2022 Dec 22;13:1026448. doi: 10.3389/fneur.2022.1026448. eCollection 2022. Front Neurol. 2022. PMID: 36619915 Free PMC article. - Chemokine CCL2 induces apoptosis in cortex following traumatic brain injury.
Liu S, Zhang L, Wu Q, Wu Q, Wang T. Liu S, et al. J Mol Neurosci. 2013 Nov;51(3):1021-9. doi: 10.1007/s12031-013-0091-8. Epub 2013 Aug 11. J Mol Neurosci. 2013. PMID: 23934512 - Craniotomy: true sham for traumatic brain injury, or a sham of a sham?
Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O'Neill JT, Grunberg NE, Dalgard CL, Frank JA, Watson WD. Cole JT, et al. J Neurotrauma. 2011 Mar;28(3):359-69. doi: 10.1089/neu.2010.1427. J Neurotrauma. 2011. PMID: 21190398 Free PMC article.
References
- Marshall L. Head injury: recent past, present and future. Neurosurgery. 2000;47:546–561. - PubMed
- CDC (Centers for Disease Control and Prevention) Facts about traumatic brain injury http://www.cdc.gov/ncipc/tbi/FactSheets/TBI_Fact_Sheets.htm Accessed March 15, 2008.
- Murray C, Lopez A. Global mortality, disability and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. - PubMed
- National Center for Injury Prevention and Control Epidemiology of traumatic brain injury in the United States. 1999.
Publication types
MeSH terms
Substances
Grants and funding
- AG013939/AG/NIA NIH HHS/United States
- R01 NS056051/NS/NINDS NIH HHS/United States
- NS056051/NS/NINDS NIH HHS/United States
- KO8 NS044998/NS/NINDS NIH HHS/United States
- R01 AG031311/AG/NIA NIH HHS/United States
- K08 NS044998/NS/NINDS NIH HHS/United States
- R37 AG013939/AG/NIA NIH HHS/United States
- R01 AG013939/AG/NIA NIH HHS/United States
- T32 AG000260/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources