Metabolism-independent sugar sensing in central orexin neurons - PubMed (original) (raw)
. 2008 Oct;57(10):2569-76.
doi: 10.2337/db08-0548. Epub 2008 Jun 30.
Affiliations
- PMID: 18591392
- PMCID: PMC2551664
- DOI: 10.2337/db08-0548
Metabolism-independent sugar sensing in central orexin neurons
J Antonio González et al. Diabetes. 2008 Oct.
Abstract
Objective: Glucose sensing by specialized neurons of the hypothalamus is vital for normal energy balance. In many glucose-activated neurons, glucose metabolism is considered a critical step in glucose sensing, but whether glucose-inhibited neurons follow the same strategy is unclear. Orexin/hypocretin neurons of the lateral hypothalamus are widely projecting glucose-inhibited cells essential for normal cognitive arousal and feeding behavior. Here, we used different sugars, energy metabolites, and pharmacological tools to explore the glucose-sensing strategy of orexin cells.
Research design and methods: We carried out patch-clamp recordings of the electrical activity of individual orexin neurons unambiguously identified by transgenic expression of green fluorescent protein in mouse brain slices. RESULTS- We show that 1) 2-deoxyglucose, a nonmetabolizable glucose analog, mimics the effects of glucose; 2) increasing intracellular energy fuel production with lactate does not reproduce glucose responses; 3) orexin cell glucose sensing is unaffected by glucokinase inhibitors alloxan, d-glucosamine, and N-acetyl-d-glucosamine; and 4) orexin glucosensors detect mannose, d-glucose, and 2-deoxyglucose but not galactose, l-glucose, alpha-methyl-d-glucoside, or fructose.
Conclusions: Our new data suggest that behaviorally critical neurocircuits of the lateral hypothalamus contain glucose detectors that exhibit novel sugar selectivity and can operate independently of glucose metabolism.
Figures
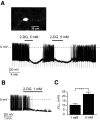
FIG. 1.
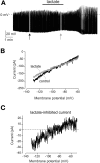
Effects of 2-deoxyglucose on the membrane potential of orexin neurons. A: A GFP-expressing orexin neuron identified in brain slice by epifluorescence (full description and validation of the GFP-labeling method is given by Burdakov et al. [11]) (top). Effects of 5 mmol/l bath-applied 2-deoxyglucose (2-DG) on membrane potential and resistance (resistance monitored as voltage deflections in response to hyperpolarizing current injections, see
research design and methods
) (bottom). B: Effect of 1 mmol/l bath-applied 2-deoxyglucose on membrane potential. C: Magnitudes of membrane potential hyperpolarization (Δ_V_m) induced by 1 and 5 mmol/l 2-deoxyglucose. ***P < 0.005, values are means ± SE, n = 4 cells for each condition.
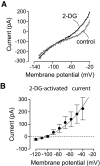
FIG. 2.
Effects of 2-deoxyglucose on the membrane currents of orexin neurons. Whole-cell voltage-clamp recordings. A: Effect of 2-deoxyglucose on membrane current-voltage relationships. B: Current-voltage relationship of the net current activated by 2-deoxyglucose. The line is a fit of the GHK equation to the data (see
research design and methods
). Values are means ± SE, n = 4 cells.
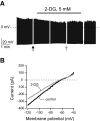
FIG. 3.
Effects of 2-deoxyglucose on cortical neurons. A: Effect of 2-deoxyglucose on the membrane potential. Breaks in the trace correspond to where the recording was interrupted to expose the cell to voltage-clamp ramps (see
research design and methods
). Arrows show where the voltage-clamp ramps shown in B were taken. B: Effect of 2-deoxyglucose on membrane current-voltage relationship of the cell shown in A. No activation of K+ current is observed, but 2-deoxyglucose inhibits a current with a reversal potential of about −50 mV.
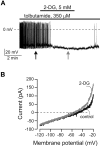
FIG. 4.
Effects of 2-deoxyglucose on orexin neurons in the presence of tolbutamide. A: Effect of 2-deoxyglucose on the membrane potential in the presence of intracellular (pipette) tolbutamide. Arrows show where the voltage-clamp ramps shown in B were recorded. B: Effect of 2-deoxyglucose on membrane current-voltage relationships of the cell shown in A.
FIG. 5.
Effects of lactate on orexin neurons. A: Effect of lactate on the membrane potential. Arrows show where voltage-clamp ramps shown in B were taken. B: Effect of lactate on the membrane current-voltage relationship of the cell shown in A. C: Current-voltage relationship of the net current inhibited by lactate.
FIG. 6.
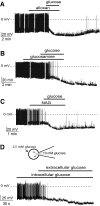
Effects of glucokinase inhibitors and intracellular glucose on the glucose response of orexin neurons. A: Effect of 5 mmol/l glucose on the membrane potential in the presence of 10 mmol/l bath alloxan. B: Effect of 5 mmol/l glucose on the membrane potential in the presence of 10 mmol/l bath
d
-glucosamine. C: Effect of 5 mmol/l glucose on the membrane potential in the presence of 4 mmol/l bath _N_-acetyl-
d
-glucosamine (NAG). D: Cartoon showing experimental protocol (top). Effect on the membrane potential of 10 mmol/l interacellular glucose and 2.5 mmol/l extracellular glucose (bottom).
FIG. 7.
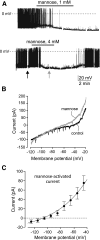
Effects of mannose on orexin neurons. A: Effect of 1 mmol/l mannose on the membrane potential (top). Effect of 4 mmol/l mannose on the membrane potential (bottom). Arrows show where membrane ramps shown in B were taken. B: Effect of mannose on membrane current-voltage relationships of the cell shown in A (bottom). C: Current-voltage relationship of the net current activated by mannose. The line is a fit of the GHK equation to the data (see
research design and methods
). Values are means ± SE, n = 6 cells.
FIG. 8.
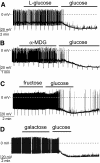
Sugars that were not effective in hyperpolarizing orexin neurons. A: Lack of effect of 5 mmol/l
l
-glucose on the membrane potential and resistance, followed by normal response to 5 mmol/l glucose. B: Lack of effect of 5 mmol/l α-methyl-
d
-glucoside (α-MDG) on the membrane potential and resistance, followed by normal response to 5 mmol/l glucose. C: Lack of effect of 5 mmol/l fructose on the membrane potential and resistance, followed by normal response to 5 mmol/l glucose. D: Lack of effect of 5 mmol/l galactose on the membrane potential, followed by normal response to 5 mmol/l glucose.
Similar articles
- Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect.
Liu ZW, Gao XB. Liu ZW, et al. J Neurophysiol. 2007 Jan;97(1):837-48. doi: 10.1152/jn.00873.2006. Epub 2006 Nov 8. J Neurophysiol. 2007. PMID: 17093123 Free PMC article. - Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels.
Venner A, Karnani MM, Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Venner A, et al. J Physiol. 2011 Dec 1;589(Pt 23):5701-8. doi: 10.1113/jphysiol.2011.217000. Epub 2011 Oct 17. J Physiol. 2011. PMID: 22005675 Free PMC article. - ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal.
Parsons MP, Hirasawa M. Parsons MP, et al. J Neurosci. 2010 Jun 16;30(24):8061-70. doi: 10.1523/JNEUROSCI.5741-09.2010. J Neurosci. 2010. PMID: 20554857 Free PMC article. - Local network regulation of orexin neurons in the lateral hypothalamus.
Burt J, Alberto CO, Parsons MP, Hirasawa M. Burt J, et al. Am J Physiol Regul Integr Comp Physiol. 2011 Sep;301(3):R572-80. doi: 10.1152/ajpregu.00674.2010. Epub 2011 Jun 22. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21697524 Review. - Dissociation between sensing and metabolism of glucose in sugar sensing neurones.
Gonzàlez JA, Reimann F, Burdakov D. Gonzàlez JA, et al. J Physiol. 2009 Jan 15;587(1):41-8. doi: 10.1113/jphysiol.2008.163410. Epub 2008 Nov 3. J Physiol. 2009. PMID: 18981030 Free PMC article. Review.
Cited by
- Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area.
González JA, Jensen LT, Fugger L, Burdakov D. González JA, et al. Eur J Neurosci. 2012 May;35(9):1426-32. doi: 10.1111/j.1460-9568.2012.08057.x. Epub 2012 Apr 16. Eur J Neurosci. 2012. PMID: 22507526 Free PMC article. - Lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNFα) blunt the response of Neuropeptide Y/Agouti-related peptide (NPY/AgRP) glucose inhibited (GI) neurons to decreased glucose.
Hao L, Sheng Z, Potian J, Deak A, Rohowsky-Kochan C, Routh VH. Hao L, et al. Brain Res. 2016 Oct 1;1648(Pt A):181-192. doi: 10.1016/j.brainres.2016.07.035. Epub 2016 Jul 26. Brain Res. 2016. PMID: 27473896 Free PMC article. - Multiple hypothalamic circuits sense and regulate glucose levels.
Karnani M, Burdakov D. Karnani M, et al. Am J Physiol Regul Integr Comp Physiol. 2011 Jan;300(1):R47-55. doi: 10.1152/ajpregu.00527.2010. Epub 2010 Nov 3. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21048078 Free PMC article. Review. - Stimulation of orexin/hypocretin neurones by thyrotropin-releasing hormone.
González JA, Horjales-Araujo E, Fugger L, Broberger C, Burdakov D. González JA, et al. J Physiol. 2009 Mar 15;587(Pt 6):1179-86. doi: 10.1113/jphysiol.2008.167940. Epub 2009 Feb 9. J Physiol. 2009. PMID: 19204048 Free PMC article. - Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms.
Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG Jr. Goforth PB, et al. J Neurosci. 2014 Aug 20;34(34):11405-15. doi: 10.1523/JNEUROSCI.5167-13.2014. J Neurosci. 2014. PMID: 25143620 Free PMC article.
References
- Saper CB, Scammell TE, Lu J: Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257–1263, 2005 - PubMed
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW: Central nervous system control of food intake and body weight. Nature 443:289–295, 2006 - PubMed
- Routh VH: Glucose-sensing neurons: are they physiologically relevant? Physiol Behav 76:403–413, 2002 - PubMed
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B: Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol 207:1146–1154, 1964 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources