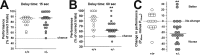Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components - PubMed (original) (raw)
. 2008 Jul 2;28(27):6872-83.
doi: 10.1523/JNEUROSCI.1815-08.2008.
Madeleine A Johnson, Michael D Lieberman, Rose E Goodchild, Scott Schobel, Nicole Lewandowski, Gorazd Rosoklija, Ruei-Che Liu, Jay A Gingrich, Scott Small, Holly Moore, Andrew J Dwork, David A Talmage, Lorna W Role
Affiliations
- PMID: 18596162
- PMCID: PMC2728592
- DOI: 10.1523/JNEUROSCI.1815-08.2008
Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components
Ying-Jiun J Chen et al. J Neurosci. 2008.
Abstract
Neuregulin-1 (Nrg1)/erbB signaling regulates neuronal development, migration, myelination, and synaptic maintenance. The Nrg1 gene is a schizophrenia susceptibility gene. To understand the contribution of Nrg1 signaling to adult brain structure and behaviors, we studied the regulation of type III Nrg1 expression and evaluated the effect of decreased expression of the type III Nrg1 isoforms. Type III Nrg1 is transcribed by a promoter distinct from those for other Nrg1 isoforms and, in the adult brain, is expressed in the medial prefrontal cortex, ventral hippocampus, and ventral subiculum, regions involved in the regulation of sensorimotor gating and short-term memory. Adult heterozygous mutant mice with a targeted disruption for type III Nrg1 (Nrg1(tm1.1Lwr+/-)) have enlarged lateral ventricles and decreased dendritic spine density on subicular pyramidal neurons. Magnetic resonance imaging of type III Nrg1 heterozygous mice revealed hypofunction in the medial prefrontal cortex and the hippocampal CA1 and subiculum regions. Type III Nrg1 heterozygous mice also have impaired performance on delayed alternation memory tasks, and deficits in prepulse inhibition (PPI). Chronic nicotine treatment eliminated differences in PPI between type III Nrg1 heterozygous mice and their wild-type littermates. Our findings demonstrate a role of type III Nrg1 signaling in the maintenance of corticostriatal components and in the neural circuits involved in sensorimotor gating and short-term memory.
Figures
Figure 1.
Neuregulin 1 gene and protein structures and the transcriptional start sites for type I and type III Nrg1 isoforms. A, The Nrg1 gene structure on mouse chromosome 8 (top) and the domain organization of three major types of Nrg1 proteins (bottom). Top, The colored blocks indicate individual exons. The approximate sizes of the introns are also indicated (not drawn to scale). All Nrg1 proteins share the EGF-like domain necessary for binding ErbB receptors. Each major type of Nrg1 protein is encoded by a distinct N-terminal exon: exon 2 (type I), exon 1 (type II), and exon 7 (type III), respectively. Three additional 5′ exons found in humans that encode type IV, V, and VI are shown as hatched boxes. C-terminal to the EGF domain, Nrg1 proteins either exist as soluble (β3) or transmembrane proteins (the β1a variant is depicted). Nrg1 heterozygous mutant mice that are described in supplemental Table 2 (available at
as supplemental material) are labeled to indicate regions (isoforms) that are disrupted in each mutant mouse. S, Spacer; CRD, cysteine-rich domain; EGF, EGF-like domain; TM, transmembrane domain. B, Transcriptional start sites for type I and type III Nrg1 mRNA were identified by sequencing multiple PCR products amplified using a standard 5′-RACE protocol. The identified 5′ ends of type I (left) and type III (right) transcripts are underlined and colored (red and blue, respectively). All type I products originated at nucleotide −542 relative to the translational initiation codon in exon 2 (bold ATG). Four distinct 5′ ends for type III transcripts were identified 593, 705, 716, and 722 nt 5′ to the first coding ATG in exon 7 (the black letters indicate noncoding sequences, and the colored letters indicate coding sequences). The dashed lines with arrows pointing the orientation indicate primers (I-a, -b, -c; CRD-a, -b, -c) used in 5′-RACE (see Materials and Methods). The sequence information is extracted from Celera locus cra_cmgc_G 307972 BP.
Figure 2.
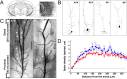
Expression of type III Nrg1 in the brain. In situ hybridization with a sense (A, D) or an antisense RNA probe (B, C, E, F) specific for type III Nrg1 revealed prominent expression (arrowheads) in corticolimbic regions of perinatal mouse brains including the following: the medial prefrontal cortex, anterior cingulate (Cg), prelimbic cortex (PrL), infralimbic cortex (IL), and forcepts minor of corpus callosum (fmi) (B, C, C′); hippocampal formation, particularly CA3 and ventral subiculum (vSUB) (E, F, F′). C and F are enlarged views of the boxes delineated in B and E. The insets (C′, F′) in C and F are enlarged views of areas delineated by white boxes. G, ErbB4 mRNA is expressed throughout the ventral striatum, which receives type III Nrg1-positive projections from PFC and ventral hippocampus. ac, Anterior commissure. Scale bars: B (for A, B), E (for D, E), 800 μm; C, F, G, 200 μm; C′, F′, 20 μm.
Figure 3.
Enlarged lateral ventricles in type III Nrg1 heterozygous mice. A, Diagram of coronal brain section, landmarks, and areas of lateral ventricle measured from wild-type (+/+) and type III Nrg1 heterozygous (+/−) sibling pairs. Scale bar: 300 μm. cc, Corpus callosum; LV, lateral ventricle; ac, anterior commissure. B, C, Representative results obtained from adult sibling pairs previously tested in T-maze and prepulse inhibition assays (Figs. 7, 8_A_). B, Hemisections from representative anterior regions measured. Top, Wild type (+/+); bottom, heterozygote (+/−). Hipp, Hippocampus; fi, fimbria. C, LV volume (in cubic micrometers) calculated from serial coronal sections and plotted from most anterior (S1) to posterior (S10) regions of wild-type (□) versus heterozygous (■) mice. D, Plot of LV volume (at level of S3) from 13 wild-type and 18 heterozygous mice (age-matched +/+ and +/− cases are connected with lines). Note that _y_-axis is discontinuous. There was a statistically significant difference of LV volume between wild-type and heterozygous animals (ANOVA for LV volume after log transformation, F(1,29) = 6.37, _p_genotype < 0.02). *p < 0.05.
Figure 4.
Type III Nrg1 heterozygous mice have decreased spine densities within proximal regions of apical dendrites of hippocampal pyramidal neurons compared with that of wild-type littermates. A, Diagram of a section (bregma, 3.40–3.64) containing the ventral subiculum (diagonal crossed lines) and a representative coronal section (Golgi stain) from which the pyramidal neurons were chosen (dashed line) and analyzed. Scale bar, 800 μm. B, Two wild-type neurons (+/+) and two heterozygous neurons (+/−) traced by Neurolucida program and reconstructed as three-dimensional structures are shown here as two-dimensional. Scale bar, 100 μm. C, Montages of photomicrographs of apical dendrites of a pyramidal neuron from +/+ or +/− animal. The proximal and distal parts of the apical dendrites are indicated (shown here are dendrites 50∼210 μm away from the center of the cell body). There are many more spines (examples indicated by white arrows) on dendrites from +/+ than from +/− mice. Scale bar, 10 μm. D, Spine densities were plotted against increasing shell radius from the center of the soma. The results are from 30 neurons of five +/+ (shown in blue) and 44 neurons of five +/− (shown in red) animals. Heterozygous mice have significantly lower spine densities at the 50–200 shell radius compared with +/+ animals (nonparametric Kolmogorov–Smirnov test; *_p_genotype < 0.05 and **_p_genotype < 0.01, respectively). Error bars indicate SEM.
Figure 5.
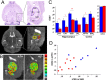
Type III Nrg1 heterozygous mice have lower function in subregions of hippocampus and in the medial prefrontal cortex. A, Horizontal brain sections (Paxinos and Franklin, 2004) (top panels) at the level of hippocampal subregions (marked and an enlarged view at the right panel): the entorhinal cortex (EC), the CA3 and CA1 subfields, the dentate gyrus (DG), and the subiculum (SUB). T2′ axial MR images at similar anatomical positions as the top panels are shown at the bottom panels. Individual areas are circled according to their respective anatomical locations and used to determine the rCBV for each region. B, MR images of one representative wild-type animal and one heterozygous animal (+/+ and +/−) with the hippocampal subregions color coded such that warmer colors reflect higher rCBV values. Note the lower rCBV observed in the CA1 and subiculum subfields of the heterozygous, and higher rCBV observed in the wild-type animals. C, Basal measures of rCBV, a hemodynamic correlate of oxygen metabolism, were obtained from each hippocampal subregion, as well as medial prefrontal cortex, sensory and motor cortex, and the nucleus accumbens. Global decreases of rCBV were found in the CA1, subiculum, medial prefontal cortex, and to a lesser degree to the CA3 subfield in heterozygous mice (red) compared with their wild-type (blue) littermates (n = 6 +/+ and 9 +/−; ANOVA test; *_p_genotype < 0.05 and **_p_genotype < 0.01). Error bars indicate SEM. D, Subiculum functions are correlated with medial prefrontal function. Blue indicates +/+, and red indicates +/− animals.
Figure 6.
Locomotor activity of age-matched wild-type and type III Nrg1 heterozygous mice. A, Locomotor activity (mean ± SEM) was measured as total distance traveled (in centimeters). B, Total rears, total jumps, and total stereotypy in a novel open field. Seventeen WT mice (+/+) versus 21 type III Nrg1 heterozygous (+/−) mice were indistinguishable in general activity patterns within 30 min of being placed in the novel open field (5 min bins) (ANOVA: F(1,36) = 0.94, _p_genotype = 0.34).
Figure 7.
Type III Nrg1 heterozygous mice have impaired delayed alternation memory performance. A, B, Performance (percentage of correct trials) of 18 wild-type (+/+) and 22 heterozygous (+/−) animals in a continuous delayed alternation test using 15 s (A) or 60 s (B) as the delay time. Each point represents the average performance of 2 d testing from individual animals. The bold lines indicate the median (50th percentile). The dashed line indicates chance performance. There is a significant deterioration in performance of +/− animals with 60 s delay (Kolmogorov–Smirnov test, χ2 = 11.1, _p_genotype < 0.008), whereas there is no difference between the performance of +/+ and +/− mice with brief intertrial intervals (15 s delay: K–S test, χ2 = 2.0, _p_genotype = 0.72). **_p_ < 0.01. **_C_**, Scatter plots of the change in performance at 15 versus 60 s delay times for each animal. Positive values reflect better performance at 60 s compared with that at 15 s; negative values reflect worse performance at 60 versus 15 s. In +/+ animals, increasing the intertrial interval to 60 s resulted in little change in performance (_p_delay time > 0.8 for wild type). In contrast, the same intertrial interval shift in +/− animals resulted in decreased performance in 17 of the 22 +/− mice. Heterozygote animals had a significant decrease in performance with increased intertrial time (Kolmogorov–Smirnov test: χ2 = 11.3; _p_delay time < 0.003). **p < 0.01.
Figure 8.
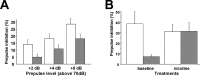
Type III Nrg1 heterozygous mice have prepulse inhibition deficits that are not seen if they have been treated with nicotine. A, PPI (mean ± SE) for wild-type (open bar; n = 13) and type III Nrg1 heterozygous (filled bar; n = 22) mice (4–8 months of age). PPI was calculated as percentage change between the responses to the startle stimuli with and without a prepulse. The PPI is presented at each prepulse intensity (2, 4, and 8 dB above the background of 70 dB, as indicated by +2, +4, +8 dB). A mixed-model, repeated-measure ANOVA indicated a statistically significant effect of prepulse intensity (F(2,158) = 29.03; p < 0.0001) and that there was a significant difference between genotypes (F(1,79) = 4.93; _p_genotype < 0.03). B, Bar plot of PPI in wild-type and type III Nrg1 heterozygous mice (n = 6/genotype) before (baseline) and after 7 weeks of chronic nicotine treatment (nicotine; 200 μg/ml). Note that this experiment was run with a separate cohort of mice with the same prepulse and startle stimuli as used in A. Therefore, the result of the baseline PPI in both genotypes was an independent replication of A to detect the effects of chronic nicotine in both genotypes. A mixed ANOVA revealed a significant genotype by nicotine treatment effect (F(1,10) = 5.63; p < 0.04). The effects of genotype (F(1,10) = 2.8; p = 0.12) and nicotine (F(1,10) = 1.54; p = 0.24) were not significant. Before, but not after nicotine, the heterozygous mice had significantly reduced PPI values (before, p < 0.05; after, p = 0.9; Student's t test with Scheffé's correction for multiple comparisons).
Similar articles
- Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice.
Nason MW Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Nason MW Jr, et al. Neuropsychopharmacology. 2011 Jan;36(2):488-96. doi: 10.1038/npp.2010.180. Epub 2010 Oct 6. Neuropsychopharmacology. 2011. PMID: 20927045 Free PMC article. - Behavioral, Neurophysiological, and Synaptic Impairment in a Transgenic Neuregulin1 (NRG1-IV) Murine Schizophrenia Model.
Papaleo F, Yang F, Paterson C, Palumbo S, Carr GV, Wang Y, Floyd K, Huang W, Thomas CJ, Chen J, Weinberger DR, Law AJ. Papaleo F, et al. J Neurosci. 2016 Apr 27;36(17):4859-75. doi: 10.1523/JNEUROSCI.4632-15.2016. J Neurosci. 2016. PMID: 27122041 Free PMC article. - Partial genetic deletion of neuregulin 1 modulates the effects of stress on sensorimotor gating, dendritic morphology, and HPA axis activity in adolescent mice.
Chohan TW, Boucher AA, Spencer JR, Kassem MS, Hamdi AA, Karl T, Fok SY, Bennett MR, Arnold JC. Chohan TW, et al. Schizophr Bull. 2014 Nov;40(6):1272-84. doi: 10.1093/schbul/sbt193. Epub 2014 Jan 17. Schizophr Bull. 2014. PMID: 24442851 Free PMC article. - Impact of neuregulin-1 on the pathophysiology of schizophrenia in human post-mortem studies.
Schmitt A, Parlapani E, Gruber O, Wobrock T, Falkai P. Schmitt A, et al. Eur Arch Psychiatry Clin Neurosci. 2008 Nov;258 Suppl 5:35-9. doi: 10.1007/s00406-008-5019-x. Eur Arch Psychiatry Clin Neurosci. 2008. PMID: 18985292 Review. - Mechanisms of neuregulin action.
Talmage DA. Talmage DA. Novartis Found Symp. 2008;289:74-84; discussion 84-93. doi: 10.1002/9780470751251.ch6. Novartis Found Symp. 2008. PMID: 18497096 Free PMC article. Review.
Cited by
- Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders.
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Schobel SA, et al. Arch Gen Psychiatry. 2009 Sep;66(9):938-46. doi: 10.1001/archgenpsychiatry.2009.115. Arch Gen Psychiatry. 2009. PMID: 19736350 Free PMC article. - Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects.
van den Buuse M. van den Buuse M. Schizophr Bull. 2010 Mar;36(2):246-70. doi: 10.1093/schbul/sbp132. Epub 2009 Nov 9. Schizophr Bull. 2010. PMID: 19900963 Free PMC article. Review. - Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models.
Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Jaaro-Peled H, et al. Schizophr Bull. 2010 Mar;36(2):301-13. doi: 10.1093/schbul/sbp133. Epub 2009 Nov 10. Schizophr Bull. 2010. PMID: 19903746 Free PMC article. Review. - Neuregulin 1 Type I Overexpression Is Associated with Reduced NMDA Receptor-Mediated Synaptic Signaling in Hippocampal Interneurons Expressing PV or CCK.
Kotzadimitriou D, Nissen W, Paizs M, Newton K, Harrison PJ, Paulsen O, Lamsa K. Kotzadimitriou D, et al. eNeuro. 2018 May 8;5(2):ENEURO.0418-17.2018. doi: 10.1523/ENEURO.0418-17.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29740596 Free PMC article. - Transient overexposure of neuregulin 3 during early postnatal development impacts selective behaviors in adulthood.
Paterson C, Law AJ. Paterson C, et al. PLoS One. 2014 Aug 5;9(8):e104172. doi: 10.1371/journal.pone.0104172. eCollection 2014. PLoS One. 2014. PMID: 25093331 Free PMC article.
References
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. - PubMed
- Aylward RL, Totterdell S. Neurons in the ventral subiculum, amygdala and entorhinal cortex which project to the nucleus accumbens: their input from somatostatin-immunoreactive boutons. J Chem Neuroanat. 1993;6:31–42. - PubMed
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. - PubMed
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DA019941/DA/NIDA NIH HHS/United States
- NS29071/NS/NINDS NIH HHS/United States
- L30 MH075110/MH/NIMH NIH HHS/United States
- P50 MH066171/MH/NIMH NIH HHS/United States
- R01 NS029071/NS/NINDS NIH HHS/United States
- DA019941/DA/NIDA NIH HHS/United States
- R21 DA019941-02/DA/NIDA NIH HHS/United States
- R01 MH064168/MH/NIMH NIH HHS/United States
- R01 NS029071-18/NS/NINDS NIH HHS/United States
- P50 MH66171-01/MH/NIMH NIH HHS/United States
- R01 NS029071-17/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous