Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling - PubMed (original) (raw)
Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling
Bryan W Luikart et al. J Neurosci. 2008.
Abstract
Synapse formation requires contact between dendrites and axons. Although this process is often viewed as axon mediated, dendritic filopodia may be actively involved in mediating synaptogenic contact. Although the signaling cues underlying dendritic filopodial motility are mostly unknown, brain-derived neurotrophic factor (BDNF) increases the density of dendritic filopodia and conditional deletion of tyrosine receptor kinase B (TrkB) reduces synapse number in vivo. Here, we report that TrkB associates with dendritic growth cones and filopodia, mediates filopodial motility, and does so via the phosphoinositide 3 kinase (PI3K) pathway. We used genetic and pharmacological manipulations of mouse hippocampal neurons to assess signaling downstream of TrkB. Conditional knock-out of two downstream negative regulators of TrkB signaling, Pten (phosphatase with tensin homolog) and Nf1 (neurofibromatosis type 1), enhanced filopodial motility. This effect was PI3K-dependent and correlated with synaptic density. Phosphatidylinositol 3,4,5-trisphosphate (PIP3) was preferentially localized in filopodia and this distribution was enhanced by BDNF application. Thus, intracellular control of filopodial dynamics converged on PI3K activation and PIP3 accumulation, a cellular paradigm conserved for chemotaxis in other cell types. Our results suggest that filopodial movement is not random, but responsive to synaptic guidance molecules.
Figures
Figure 1.
TrkB-YFP puncta are rapidly transported within primary dendrites and stable in dendritic growth cones and filopodia. A, B, We observed TrkB puncta being rapidly transported in both the retrograde (A, arrowheads) and anterograde (B, arrowheads) directions. Within primary dendrites, TrkB puncta traveled at an average velocity of 1.008 ± 0.082 μm/s in the anterograde and 0.988 ± 0.071 μm/s in the retrograde direction (mean ± SEM; n = 50 and 51 puncta from 6 cells, respectively). C, D, At longer time intervals (1 and 5 min), we resolved that TrkB puncta were less mobile in immature dendritic branches, growth cones, and filopodia (arrowheads). In these structures, TrkB puncta moved at an average velocity of 0.015 ± 0.002 μm/s (mean ± SEM; n = 85 puncta from 3 cells).
Figure 2.
Dendritic filopodial motility quantitation. A, Time-lapse images of dendrites from neurons expressing GFP. B, Filopodial motility was calculated as the average of the absolute value of the sum of changed filopodial lengths per dendrite length every 5 min for a total of 30 min. The colored dots represent the tip of each filopodium and the corresponding line shows the distance the filopodium travels over time. For this example, the dendritic segment is 17.56 μm long. The average distance each filopodium moves every 5 min is 2.91 μm, and the motility is 0.166 (2.91/17.56).
Figure 3.
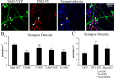
Synapse density in TrkB, Nf1, and Pten mutant hippocampal neurons. A, Immunohistochemistry for PSD-95 (red) and synaptophysin (blue) in neurons expressing the TrkB-YFP fusion protein (green). Synapses were identified for quantitation as points at which the red, green, and blue signals overlapped (A, arrowheads). Synaptic density was measured for TrkB-YFP (WT; n = 80 neurons), TrkB Y490F-YFP (n = 79), TrkB Y785F-YFP (n = 77), TrkB Y490/785F-YFP (n = 78), and TrkB K538N-YFP (KD, n = 77). The Y490F, Y490/785F, and K538N mutations resulted in a significant decrease in synapse number when compared with TrkB-YFP. B, However, TrkB Y785F-YFP was not significantly different from TrkB-YFP. Synapse density was measured for neurons from Nf1 flox and Pten flox animals transfected with GFP or GFP-cre. C, The deletion of both Nf1 (n = 30 neurons) and Pten (n = 30) resulted in a significant increase in synaptic density when compared with GFP (n = 34; p values calculated using two-sample equal variance t test).
Figure 4.
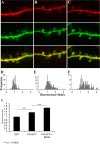
BDNF-dependent accumulation of PH-GFP in dendritic filopodia. A–C, Representative images of dendritic segments expressing GFP and mCherry (A), PH-GFP and mCherry (B), and PH-GFP and mCherry from BDNF-treated (50 ng/ml for 4–6 h) slices (C). The distribution of GFP fluorescence in filopodia relative to dendrites was quantified as the filopodial 488/568 ratio divided by the dendritic 488/568 ratio (fluorescence index). Thus, numbers >1 indicate increased relative GFP fluorescence in the filopodia versus dendrites. D–F, The distribution of these values was plotted for GFP (D; n = 79 filopodia), PH-GFP (E; n = 230 filopodia), and BDNF treated PH-GFP (F; n = 151 filopodia). The equal distribution of GFP and mCherry between dendrites and filopodia is indicated by the distribution of the fluorescence index around 1. The shift in this distribution toward numbers >1 in the PH-GFP condition indicates accumulation of PIP3 in dendritic filopodia. Application of BDNF further increased the filopodial PIP3 levels when compared with untreated cells (p values calculated using the Newman–Keuls test).
Similar articles
- Local calcium transients regulate the spontaneous motility of dendritic filopodia.
Lohmann C, Finski A, Bonhoeffer T. Lohmann C, et al. Nat Neurosci. 2005 Mar;8(3):305-12. doi: 10.1038/nn1406. Epub 2005 Feb 13. Nat Neurosci. 2005. PMID: 15711541 - BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses.
Tyler WJ, Pozzo-Miller LD. Tyler WJ, et al. J Neurosci. 2001 Jun 15;21(12):4249-58. doi: 10.1523/JNEUROSCI.21-12-04249.2001. J Neurosci. 2001. PMID: 11404410 Free PMC article. - The regulation of cell motility and chemotaxis by phospholipid signaling.
Kölsch V, Charest PG, Firtel RA. Kölsch V, et al. J Cell Sci. 2008 Mar 1;121(Pt 5):551-9. doi: 10.1242/jcs.023333. J Cell Sci. 2008. PMID: 18287584 Free PMC article. Review. - Activity-dependent dendritic release of BDNF and biological consequences.
Kuczewski N, Porcher C, Lessmann V, Medina I, Gaiarsa JL. Kuczewski N, et al. Mol Neurobiol. 2009 Feb;39(1):37-49. doi: 10.1007/s12035-009-8050-7. Epub 2009 Jan 22. Mol Neurobiol. 2009. PMID: 19156544 Free PMC article. Review.
Cited by
- BDNF-TrkB Signaling in Mitochondria: Implications for Neurodegenerative Diseases.
K Soman S, Swain M, Dagda RK. K Soman S, et al. Mol Neurobiol. 2024 Jul 19. doi: 10.1007/s12035-024-04357-4. Online ahead of print. Mol Neurobiol. 2024. PMID: 39030441 Review. - The Role of Inhaled Estradiol and Myrtenol, Alone and in Combination, in Modulating Behavioral and Functional Outcomes Following Traumatic Experimental Brain Injury: Hemodynamic, Molecular, Histological and Behavioral Study.
Rajizadeh MA, Khaksari M, Bejeshk MA, Amirkhosravi L, Jafari E, Jamalpoor Z, Nezhadi A. Rajizadeh MA, et al. Neurocrit Care. 2023 Oct;39(2):478-498. doi: 10.1007/s12028-023-01720-6. Epub 2023 Apr 26. Neurocrit Care. 2023. PMID: 37100976 - How Are Synapses Born? A Functional and Molecular View of the Role of the Wnt Signaling Pathway.
Bonansco C, Cerpa W, Inestrosa NC. Bonansco C, et al. Int J Mol Sci. 2022 Dec 31;24(1):708. doi: 10.3390/ijms24010708. Int J Mol Sci. 2022. PMID: 36614149 Free PMC article. Review. - Ras Inhibitor Lonafarnib Rescues Structural and Functional Impairments of Synapses of Aβ1-42 Mice via α7nAChR-Dependent BDNF Upregulation.
Cai C, Wang L, Li S, Lou S, Luo JL, Fu DY, Chen T. Cai C, et al. J Neurosci. 2022 Aug 3;42(31):6090-6107. doi: 10.1523/JNEUROSCI.1989-21.2022. Epub 2022 Jun 27. J Neurosci. 2022. PMID: 35760529 Free PMC article. - Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134-mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression.
Wang G, An T, Lei C, Zhu X, Yang L, Zhang L, Zhang R. Wang G, et al. J Ginseng Res. 2022 May;46(3):376-386. doi: 10.1016/j.jgr.2021.03.005. Epub 2021 Mar 20. J Ginseng Res. 2022. PMID: 35600767 Free PMC article.
References
- Akins MR, Biederer T. Cell–cell interactions in synaptogenesis. Curr Opin Neurobiol. 2006;16:83–89. - PubMed
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. - PubMed
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. - PubMed
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH46613/MH/NIMH NIH HHS/United States
- R01 MH046613/MH/NIMH NIH HHS/United States
- F32 MH079548/MH/NIMH NIH HHS/United States
- P50 NS052606-04/NS/NINDS NIH HHS/United States
- R37NS33199/NS/NINDS NIH HHS/United States
- P50 NS 052606/NS/NINDS NIH HHS/United States
- R37 NS033199/NS/NINDS NIH HHS/United States
- P50 NS052606/NS/NINDS NIH HHS/United States
- F32MH079548/MH/NIMH NIH HHS/United States
- R37 MH046613/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous