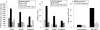Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition - PubMed (original) (raw)
Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition
D G Binion et al. Gut. 2008 Nov.
Abstract
Background: Angiogenesis, the growth of new blood vessels, is a critical homeostatic mechanism which regulates vascular populations in response to physiological requirements and pathophysiological demand, including chronic inflammation and cancer. The importance of angiogenesis in gastrointestinal chronic inflammation and cancer has been defined, as antiangiogenic therapy has demonstrated benefit in models of inflammatory bowel disease and colon cancer treatment. Curcumin is a natural product undergoing evaluation for the treatment of chronic inflammation, including inflammatory bowel disease (IBD). The effect of curcumin on human intestinal angiogenesis is not defined.
Methods: The antiangiogenic effect of curcumin on in vitro angiogenesis was examined using primary cultures of human intestinal microvascular endothelial cells (HIMECs), stimulated with vascular endothelial growth factor (VEGF).
Results: Curcumin inhibited proliferation, cell migration and tube formation in HIMECs induced by VEGF. Activation of HIMECs by VEGF resulted in enhanced expression of cyclo-oxygenase-2 (COX-2) mRNA, protein and prostaglandin E(2) (PGE(2)) production. Pretreatment of HIMECs with 10 microM curcumin as well as 1 microM NS398, a selective inhibitor of COX-2, resulted in inhibition of COX-2 at the mRNA and protein level and PGE(2) production. Similarly COX-2 expression in HIMECs was significantly inhibited by Jun N-terminal kinase (JNK; SP600125) and p38 mitogen-activated protein kinase (MAPK; SB203580) inhibitors and was reduced by p44/42 MAPK inhibitor (PD098059).
Conclusions: Taken together, these data demonstrate an important role for COX-2 in the regulation of angiogenesis in HIMECs via MAPKs. Moreover, curcumin inhibits microvascular endothelial cell angiogenesis through inhibition of COX-2 expression and PGE(2) production, suggesting that this natural product possesses antiangiogenic properties, which warrants further investigation as adjuvant treatment of IBD and cancer.
Conflict of interest statement
Competing interests: None.
Figures
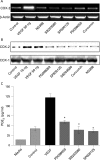
Figure 1. Effect of vascular cell adhesion factor (VEGF) on cyclo-oxygenase-1 (COX-1) and COX-2 mRNA and protein expression in human intestinal microvascular endothelial cells (HIMECs). (A) Semi-quantitative reverse transcription–PCR of COX mRNA in HIMECs demonstrates that COX-1 mRNA, but not COX-2, was constitutively expressed in HIMECs. VEGF (50 ng/ml) stimulation of HIMECs resulted in marked upregulation of COX-2 gene expression by 3 h, whereas COX-1 was unaffected. β-Actin was used as an internal loading control. (B) Western blot analysis demonstrates that VEGF enhanced COX-2 protein expression in HIMECs by 6 h, which persisted until 24 h, declining by 36 h. COX-1 protein expression was not affected by VEGF. (C) The results of ELISA for prostaglandin E2 (PGE2) and 6-keto PGF1α in HIMEC culture supernatants demonstrate that VEGF stimulation significantly increased both PGE2 and 6-keto PGF1α production in HIMECs. Data are expressed as pg/ml PG production (SD). *p<0.05 compared with control.
Figure 2. Curcumin inhibits cyclo-oxygenase-2 (COX-2) expression in human intestinal microvascular endothelial cells (HIMECs). (A) Pretreatment of HIMECs with curcumin abolished vascular endothelial growth factor (VEGF) induction of COX-2 mRNA expression in a dose-dependent fashion. (B) Consistent with the gene expression data, curcumin pretreatment of HIMECs inhibited COX-2 protein expression at 1 µM, increasing at 10 µM, while 20 µM curcumin completely abolished COX-2 expression following VEGF activation. (C) Immunofluorescence staining of HIMECs pretreated with either 10 μM curcumin or 1 μM NS398 demonstrated inhibition of COX-2 expression following VEGF activation. (D) Curcumin inhibited both prostaglandin E2 (PGE2) and 6-keto PGF1α production in HIMECs in a dose-dependent manner. Prostanoid species were assessed from HIMEC culture media using ELISA. Data were expressed as pg/ml PG production (SD). *p<0.05 compared with no stimulation; **p<0.05 compared with VEGF stimulation. (E) PGE2 and 6-keto PGF1α production were also completely inhibited by pre-treatment with 1 μM NS398. Data were expressed as pg/ml PG production (SD). *p<0.05 compared with no stimulation; **p<0.05 compared with VEGF stimulation.
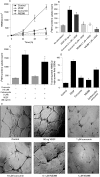
Figure 3. Curcumin inhibits growth, proliferation, migration and tube formation in human intestinal microvascular endothelial cells (HIMECs). (A) Potent angiogenic effect of vascular endothelial growth factor (VEGF) compared with no stimulation in HIMECs. Curcumin inhibited HIMEC growth at rates almost similar to those of control cells. The cyclo-oxygenase-2 (COX-2) inhibitor, NS398 was a potent inhibitor of cell growth. (B) Cellular DNA synthesis was assessed by measuring [H]thymidine uptake. [H]Thymidine uptake was significantly increased after VEGF stimulation for 15 h and was inhibited by curcumin pretreatment for 30 min in a dose-dependent manner. Assays were done in triplicate and the data are shown as mean cpm (SD). *p<0.05 compared with VEGF-stimulated HIMEC cultures. (C) The inhibitory effect of curcumin on [H]thymidine uptake was reversed by addition of carbacyclin (a prostaglandin I2 (PGI2) analogue). *p<0.05 compared with curcumin-treated HIMECs with no exogenous PG. (D) The number of HIMECs transmigrated through the filter was increased by VEGF stimulation; curcumin pretreatment of HIMECs significantly inhibited HIMEC transmigration, which was reversed by 1 μM carbacyclin. At least 15 random high-power fields (×200) per condition were counted and data were expressed as mean (SD). *p<0.05 compared with curcumin pretreatment. (E) Phase-contrast photomicrograph demonstrates the endothelial in vitro tube formation on Matrigel; the formation of capillary-like structures was inhibited by curcumin pretreatment (×40).
Figure 4. Modulation of cyclo-oxygenase-2 (COX-2) expression in human intestinal microvascular endothelial cells (HIMECs) by mitogen-activated protein kinase (MAPK) inhibitors. (A and B) Inhibition of the MAPK pathways resulted in downregulation and suppression of COX-2 mRNA and protein. Pretreatment of HIMECs with either 10 µM PD098059 (p44/42 MAPK), 5 µM SB203580 (p38 MAPK) or 10 µM SP600125 (Jun N-terminal kinase) significantly inhibited vascular endothelial growth factor (VEGF)-induced COX-2 mRNA and protein expression. (C) All three MAPK inhibitors inhibited prostaglandin E2 (PGE2) production as determined by ELISA in HIMEC culture media. *p <0.05 for inhibitors vs VEGF.
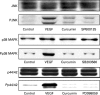
Figure 5. Curcumin inhibits the activation of mitogen-activated protein kinases (MAPKs) in vascular endothelial growth factor (VEGF)-activated human intestinal microvascular endothelial cells (HIMECs). Pretreatment of HIMECs with 10 µM curcumin following VEGF activation resulted in inhibition of p44/42 MAPK, p38 MAPK and Jun N-terminal kinase (JNK) phosphorylation.
Figure 6. Effect of curcumin on cell adhesion molecule (CAM) expression and leucocyte binding in human intestinal microvascular endothelial cells (HIMECs). (A) Radioimmunoassay analysis demonstrates that curcumin pretreatment of HIMECs followed by tumour necrosis factor α (TNFα)/lipopolysaccharide (LPS) activation completely inhibited intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule (VCAM) expression but only partially reduced E-selectin. *p <0.05. (B) Fluorescence activated cell sorting analysis of HIMECs demonstrates the similar inhibitory effect of curcumin on CAM expression. Data are expressed as mean (SD) from triplicate wells. *p <0.05. (C) Low-shear stress flow adhesion assay demonstrates that curcumin pretreatment of HIMECs resulted in inhibition of U-937 leucocyte adhesion. *p <0.05.
Similar articles
- Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells.
Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Wu G, et al. Cardiovasc Res. 2006 Feb 1;69(2):512-9. doi: 10.1016/j.cardiores.2005.09.019. Epub 2005 Dec 5. Cardiovasc Res. 2006. PMID: 16336951 - Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition.
Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG. Ogawa H, et al. FEBS Lett. 2003 Nov 6;554(1-2):88-94. doi: 10.1016/s0014-5793(03)01110-4. FEBS Lett. 2003. PMID: 14596920 - Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2.
Hernández GL, Volpert OV, Iñiguez MA, Lorenzo E, Martínez-Martínez S, Grau R, Fresno M, Redondo JM. Hernández GL, et al. J Exp Med. 2001 Mar 5;193(5):607-20. doi: 10.1084/jem.193.5.607. J Exp Med. 2001. PMID: 11238591 Free PMC article. - Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy.
Gately S, Li WW. Gately S, et al. Semin Oncol. 2004 Apr;31(2 Suppl 7):2-11. doi: 10.1053/j.seminoncol.2004.03.040. Semin Oncol. 2004. PMID: 15179620 Review. - Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis.
Gately S, Kerbel R. Gately S, et al. Prog Exp Tumor Res. 2003;37:179-92. doi: 10.1159/000071373. Prog Exp Tumor Res. 2003. PMID: 12795055 Review.
Cited by
- Curcumin Inhibits Acute Vascular Inflammation through the Activation of Heme Oxygenase-1.
Xiao Y, Xia J, Wu S, Lv Z, Huang S, Huang H, Su X, Cheng J, Ke Y. Xiao Y, et al. Oxid Med Cell Longev. 2018 Sep 20;2018:3295807. doi: 10.1155/2018/3295807. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30327711 Free PMC article. - Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin.
Binion DG, Heidemann J, Li MS, Nelson VM, Otterson MF, Rafiee P. Binion DG, et al. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G259-68. doi: 10.1152/ajpgi.00087.2009. Epub 2009 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19520742 Free PMC article. - Bioinformatics and Network Pharmacology Identify the Therapeutic Role and Potential Mechanism of Melatonin in AD and Rosacea.
Zhang H, Zhang Y, Li Y, Wang Y, Yan S, Xu S, Deng Z, Yang X, Xie H, Li J. Zhang H, et al. Front Immunol. 2021 Nov 23;12:756550. doi: 10.3389/fimmu.2021.756550. eCollection 2021. Front Immunol. 2021. PMID: 34899707 Free PMC article. - Regulation of the expression of cyclooxygenases and production of prostaglandin I₂ and E₂ in human coronary artery endothelial cells by curcumin.
Tan X, Poulose EM, Raveendran VV, Zhu BT, Stechschulte DJ, Dileepan KN. Tan X, et al. J Physiol Pharmacol. 2011 Feb;62(1):21-8. J Physiol Pharmacol. 2011. PMID: 21451206 Free PMC article. - Therapeutic potential of curcumin in gastrointestinal diseases.
Rajasekaran SA. Rajasekaran SA. World J Gastrointest Pathophysiol. 2011 Feb 15;2(1):1-14. doi: 10.4291/wjgp.v2.i1.1. World J Gastrointest Pathophysiol. 2011. PMID: 21607160 Free PMC article.
References
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–9 - PubMed
- Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development 1999;126:1149–59 - PubMed
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31 - PubMed
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999;77:527–43 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous