Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes - PubMed (original) (raw)
Clinical Trial
Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes
Lynda A Szczech et al. Kidney Int. 2008 Sep.
Abstract
Trials of anemia correction in chronic kidney disease have found either no benefit or detrimental outcomes of higher targets. We did a secondary analysis of patients with chronic kidney disease enrolled in the Correction of Hemoglobin in the Outcomes in Renal Insufficiency trial to measure the potential for competing benefit and harm from achieved hemoglobin and epoetin dose trials. In the 4 month analysis, significantly more patients in the high-hemoglobin compared to the low-hemoglobin arm were unable to achieve target hemoglobin and required high-dose epoetin-alpha. In unadjusted analyses, the inability to achieve a target hemoglobin and high-dose epoetin-alpha were each significantly associated with increased risk of a primary endpoint (death, myocardial infarction, congestive heart failure or stroke). In adjusted models, high-dose epoetin-alpha was associated with a significant increased hazard of a primary endpoint but the risk associated with randomization to the high hemoglobin arm did not suggest a possible mediating effect of higher target via dose. Similar results were seen in the 9 month analysis. Our study demonstrates that patients achieving their target had better outcomes than those who did not; and among subjects who achieved their randomized target, no increased risk associated with the higher hemoglobin goal was detected. Prospective studies are needed to confirm this relationship and determine safe dosing algorithms for patients unable to achieve target hemoglobin.
Figures
Figure 1
Cox proportional hazards models for the primary composite end point of death, coronary heart failure hospitalization, stroke, or MI.
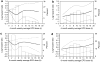
Figure 2. Association between epoetin-α dose and primary end point
(a) Among subjects randomized to the low-hemoglobin group in 4-month landmark analysis. (b) Among subjects randomized to the high-hemoglobin group in the 4-month landmark analysis. (c) Among subjects randomized to the low-hemoglobin group in 9-month landmark analysis. (d) Among subjects randomized to the high-hemoglobin group in the 9-month landmark analysis. (The line indicates the log hazard. The bar graph indicates the percent of treatment arm that fell within each dosing interval.)
Comment in
- The mortality risk associated with higher hemoglobin: is the therapy to blame?
Rosner MH, Bolton WK. Rosner MH, et al. Kidney Int. 2008 Sep;74(6):695-7. doi: 10.1038/ki.2008.263. Kidney Int. 2008. PMID: 18756292 Review.
Similar articles
- Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease.
McCullough PA, Barnhart HX, Inrig JK, Reddan D, Sapp S, Patel UD, Singh AK, Szczech LA, Califf RM. McCullough PA, et al. Am J Nephrol. 2013;37(6):549-58. doi: 10.1159/000351175. Epub 2013 May 25. Am J Nephrol. 2013. PMID: 23735819 Clinical Trial. - Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients.
Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman-Breen C, Krishnan M, Bradbury BD. Kilpatrick RD, et al. Clin J Am Soc Nephrol. 2008 Jul;3(4):1077-83. doi: 10.2215/CJN.04601007. Epub 2008 Apr 16. Clin J Am Soc Nephrol. 2008. PMID: 18417744 Free PMC article. Clinical Trial. - A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment.
Szczech LA, Barnhart HX, Sapp S, Felker GM, Hernandez A, Reddan D, Califf RM, Inrig JK, Patel UD, Singh AK. Szczech LA, et al. Kidney Int. 2010 Feb;77(3):239-46. doi: 10.1038/ki.2009.415. Epub 2009 Nov 4. Kidney Int. 2010. PMID: 19890274 Free PMC article. Clinical Trial. - Epoetin alfa therapy for patients with hematologic malignancies and mild anemia.
Straus DJ. Straus DJ. Clin Lymphoma. 2003 Aug;4 Suppl 1:S13-7. doi: 10.3816/clm.2003.s.003. Clin Lymphoma. 2003. PMID: 14556671 Review. - The relative dosing of epoetin alfa and darbepoetin alfa in chronic kidney disease.
Cremieux PY, Van Audenrode M, Lefebvre P. Cremieux PY, et al. Curr Med Res Opin. 2006 Dec;22(12):2329-36. doi: 10.1185/030079906X154024. Curr Med Res Opin. 2006. PMID: 17257447 Review.
Cited by
- Usage of the Anemia Control Model Is Associated with Reduced Hospitalization Risk in Hemodialysis.
Garbelli M, Baro Salvador ME, Rincon Bello A, Samaniego Toro D, Bellocchio F, Fumagalli L, Chermisi M, Apel C, Petrovic J, Kendzia D, Ion Titapiccolo J, Yeung J, Barbieri C, Mari F, Usvyat L, Larkin J, Stuard S, Neri L. Garbelli M, et al. Biomedicines. 2024 Sep 28;12(10):2219. doi: 10.3390/biomedicines12102219. Biomedicines. 2024. PMID: 39457532 Free PMC article. - Adiposity and Mineral Balance in Chronic Kidney Disease.
Hosain O, Clinkenbeard EL. Hosain O, et al. Curr Osteoporos Rep. 2024 Dec;22(6):561-575. doi: 10.1007/s11914-024-00884-0. Epub 2024 Oct 11. Curr Osteoporos Rep. 2024. PMID: 39394545 Review. - Analisi di impatto sul budget sanitario italiano di roxadustat per il trattamento dell’anemia da malattia renale cronica.
Bini C, Marcellusi A, Di Rienzo P, Del Vecchio L. Bini C, et al. Glob Reg Health Technol Assess. 2024 Sep 10;11:175-190. doi: 10.33393/grhta.2024.3062. eCollection 2024 Jan-Dec. Glob Reg Health Technol Assess. 2024. PMID: 39281665 Free PMC article. Italian. - The efficacy and safety of molidustat for anemia in dialysis-dependent and non-dialysis-dependent chronic kidney disease patients: A systematic review and meta-analysis.
Kang Y, Zhou M, Jin Q, Geng YL, Wang Y, Lv J. Kang Y, et al. Heliyon. 2024 May 4;10(9):e30621. doi: 10.1016/j.heliyon.2024.e30621. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765138 Free PMC article. - Stabilizing Hypoxia-Inducible Factor to Manage Anemia in Chronic Kidney Disease: From Basic Theory to Clinical Study.
Wang Y, Yu X. Wang Y, et al. Kidney Dis (Basel). 2024 Jan 3;10(2):132-142. doi: 10.1159/000536039. eCollection 2024 Apr. Kidney Dis (Basel). 2024. PMID: 38659701 Free PMC article. Review.
References
- Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. - PubMed
- Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. - PubMed
- Ma JZ, Ebben J, Xia H, et al. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10:610–619. - PubMed
- Xia H, Ebben J, Ma JZ, et al. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999;10:1309–1316. - PubMed
- Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK080094/DK/NIDDK NIH HHS/United States
- KL2 RR024127/RR/NCRR NIH HHS/United States
- K23DK075929/DK/NIDDK NIH HHS/United States
- K23 DK075929/DK/NIDDK NIH HHS/United States
- 1KL2 RR024127/RR/NCRR NIH HHS/United States
- K23 DK075929-03/DK/NIDDK NIH HHS/United States
- R01 DK080094-02/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical