Mechanics of membrane fusion - PubMed (original) (raw)
Review
Mechanics of membrane fusion
Leonid V Chernomordik et al. Nat Struct Mol Biol. 2008 Jul.
Abstract
Diverse membrane fusion reactions in biology involve close contact between two lipid bilayers, followed by the local distortion of the individual bilayers and reformation into a single, merged membrane. We consider the structures and energies of the fusion intermediates identified in experimental and theoretical work on protein-free lipid bilayers. On the basis of this analysis, we then discuss the conserved fusion-through-hemifusion pathway of merger between biological membranes and propose that the entire progression, from the close juxtaposition of membrane bilayers to the expansion of a fusion pore, is controlled by protein-generated membrane stresses.
Figures
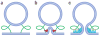
Figure 1
Fusion-through hemifusion pathway of lipid bilayer fusion. (a) (i) Pre-fusion contact. (ii) A point-like membrane protrusion minimizes the energy of the hydration repulsion between the proximal leaflets of the membranes coming into immediate contact. (iii) A hemifusion stalk with proximal leaflets fused and distal leaflets unfused. (iv) Stalk expansion yields the hemifusion diaphragm. (v) A fusion pore forms either in the hemifusion diaphragm bilayer or directly from the stalk. Dashed lines show the boundaries of the hydrophobic surfaces of monolayers. (b) Different lipids spontaneously form monolayers of different curvatures and, thus, demonstrate different effective molecular shapes. Monolayers formed by inverted cone–shaped lysophosphatidylcholine (LPC) and by cone-shaped phosphatidylethanolamine (PE) and diacylglycerol (DAG) bulge in the direction of the polar heads and in the direction of the hydrocarbon chains, respectively. Cylindrical phosphatidylcholine (PC) forms an almost flat monolayer.
Figure 2
The stalk is the key intermediate in most of the theoretical models developed with the continuous and the simulation approaches. (a) Stalk structure computed by analysis of bending, splay and tilt of the lipid molecules in the membrane monolayers with the elastic model (continuous approach). (b) Stalk structure computed by the self-consistent field model (continuous approach). Light regions indicate the areas of head groups of the bilayer. (c) Stalk structure ‘observed’ by molecular dynamics simulation of the fusion between liposomes composed of dipalmitoyl phosphatidylcholine and palmitic acid using an atomistically detailed model. Water molecules (gray) and head group atoms of the lipids are depicted as spheres; tails are shown as bonds, with gray used to distinguish water molecules originating on different sides of the fusing membranes. The coloring also distinguishes between lipid molecules coming from different leaflets of the bilayers: dipalmitoyl phosphatidylcholine molecules in the inner or outer leaflets (green and purple), and palmitic acid in the inner or outer leaflets (cyan or magenta, respectively).
Figure 3
Hypothetical pathway of biological fusion powered by protein-generated membrane stresses. (a) In the initial state, apposing membrane bilayers are separated by at least a 10–20 nm gap. The contact might involve protein fusogens themselves or be mediated by specialized tethering molecules (green shapes). (b) Fusion proteins induce local bending of membrane bilayer(s) and establish very close contact between the membranes. Generation of large membrane curvature might involve shallow insertion of amphiphilic protein domains (red shapes) into the membrane,. The highly stressed and protein-depleted tops of the bilayer bulges are primed for hemifusion and pore opening,,,. (c) Activated fusion proteins (blue shapes) might drive fusion pore expansion by assembling into an interconnected protein coat surrounding the fusion site. This membrane-associated fusion coat has an intrinsic curvature opposite to that of the budding and fission coats. The coat, bending toward its preferred curvature, deforms the underlying membrane and produces tension that drives fusion and expands the fusion pore.
Similar articles
- The mechanisms of lipid-protein rearrangements during viral infection.
Chizmadzhev YA. Chizmadzhev YA. Bioelectrochemistry. 2004 Jun;63(1-2):129-36. doi: 10.1016/j.bioelechem.2003.10.016. Bioelectrochemistry. 2004. PMID: 15110263 Review. - The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement.
Cohen FS, Melikyan GB. Cohen FS, et al. J Membr Biol. 2004 May 1;199(1):1-14. doi: 10.1007/s00232-004-0669-8. J Membr Biol. 2004. PMID: 15366419 Review. - Myomerger Induces Membrane Hemifusion and Regulates Fusion Pore Expansion.
Di Bartolo AL, Caparotta M, Polo LM, Masone D. Di Bartolo AL, et al. Biochemistry. 2024 Mar 19;63(6):815-826. doi: 10.1021/acs.biochem.3c00682. Epub 2024 Feb 13. Biochemistry. 2024. PMID: 38349279 - Greasing membrane fusion and fission machineries.
Burger KN. Burger KN. Traffic. 2000 Aug;1(8):605-13. doi: 10.1034/j.1600-0854.2000.010804.x. Traffic. 2000. PMID: 11208148 Review. - Point-like protrusion as a prestalk intermediate in membrane fusion pathway.
Efrat A, Chernomordik LV, Kozlov MM. Efrat A, et al. Biophys J. 2007 Apr 15;92(8):L61-3. doi: 10.1529/biophysj.106.103341. Epub 2007 Feb 2. Biophys J. 2007. PMID: 17277178 Free PMC article.
Cited by
- Actin dynamics switches two distinct modes of endosomal fusion in yolk sac visceral endoderm cells.
Koike S, Tachikawa M, Tsutsumi M, Okada T, Nemoto T, Keino-Masu K, Masu M. Koike S, et al. Elife. 2024 Oct 23;13:RP95999. doi: 10.7554/eLife.95999. Elife. 2024. PMID: 39441732 Free PMC article. - Lipid transport between the endoplasmic reticulum and mitochondria.
Flis VV, Daum G. Flis VV, et al. Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a013235. doi: 10.1101/cshperspect.a013235. Cold Spring Harb Perspect Biol. 2013. PMID: 23732475 Free PMC article. Review. - Local electrostatic interactions determine the diameter of fusion pores.
Guček A, Jorgačevski J, Górska U, Rituper B, Kreft M, Zorec R. Guček A, et al. Channels (Austin). 2015;9(2):96-101. doi: 10.1080/19336950.2015.1007825. Channels (Austin). 2015. PMID: 25835258 Free PMC article. - Single-virus fusion experiments reveal proton influx into vaccinia virions and hemifusion lag times.
Schmidt FI, Kuhn P, Robinson T, Mercer J, Dittrich PS. Schmidt FI, et al. Biophys J. 2013 Jul 16;105(2):420-31. doi: 10.1016/j.bpj.2013.06.016. Biophys J. 2013. PMID: 23870263 Free PMC article. - Insertion and Anchoring of HIV-1 Fusion Peptide into Complex Membrane Mimicking Human T-cell.
Zhao M, Lopes LJS, Sahni H, Yadav A, Do HN, Reddy T, López CA, Neale C, Gnanakaran S. Zhao M, et al. bioRxiv [Preprint]. 2024 Aug 4:2024.08.02.606381. doi: 10.1101/2024.08.02.606381. bioRxiv. 2024. PMID: 39131401 Free PMC article. Updated. Preprint.
References
- Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer. 2007;7:968–976. - PubMed
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources