Regulation of APC/C activators in mitosis and meiosis - PubMed (original) (raw)
Review
Regulation of APC/C activators in mitosis and meiosis
Jillian A Pesin et al. Annu Rev Cell Dev Biol. 2008.
Abstract
The anaphase-promoting complex/cyclosome (APC/C) is a multisubunit E3 ubiquitin ligase that triggers the degradation of multiple substrates during mitosis. Cdc20/Fizzy and Cdh1/Fizzy-related activate the APC/C and confer substrate specificity through complex interactions with both the core APC/C and substrate proteins. The regulation of Cdc20 and Cdh1 is critical for proper APC/C activity and occurs in multiple ways: targeted protein degradation, phosphorylation, and direct binding of inhibitory proteins. During the specialized divisions of meiosis, the activity of the APC/C must be modified to achieve proper chromosome segregation. Recent studies show that one way in which APC/C activity is modified is through the use of meiosis-specific APC/C activators. Furthermore, regulation of the APC/C during meiosis is carried out by both mitotic regulators of the APC/C as well as meiosis-specific regulators. Here, we review the regulation of APC/C activators during mitosis and the role and regulation of the APC/C during female meiosis.
Figures
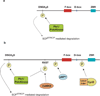
Figure 1. Regulation of Cdc20 and Cdh1 during the Cell Cycle
(top) During S and G2 phases, APC/CCdc20 is thought to be bound and inhibited by Emi1. Emi1 is degraded in prometaphase, and APC/CCdc20 becomes active against early mitotic APC/C substrates like Cyclin A and Nek2A. In prometaphase APC/CCdc20 remains inhibited from targeting Cyclin B, Securin, and other substrates by the spindle assembly checkpoint through direct binding of Mad2, Bub3, and BubR1. Upon release of the spindle assembly checkpoint, APC/CCdc20 becomes fully active. In late anaphase and G1, Cdc20 is targeted by APC/CCdh1 for degradation by the proteasome. It will not be expressed again until S phase. (bottom) Cdh1 is inhibited from associating with the core APC/C in S, G2, and M phases by phosphorylation by cyclin-dependent kinases. In Drosophila, Rca1 is thought to inhibit Cdh1 in G2, and in vertebrates Emi1 is thought to inhibit APC/CCdh1 at the G1-S transition. Recent findings suggest that APC/CCdh1 must be inhibited from targeting Securin from degradation by Rae1-Nup98 in metaphase. Dephosphorylation of Cdh1 by Cdc14 phophatase as well as a decrease in mitotic Cdk-cyclin activity leads to loss of inhibitory phosphorylation on Cdh1 in late mitosis and allows for full activation of APC/CCdh1 in late mitosis and G1. In addition, Cdh1 may be subject to ubiquitin-mediated degradation in late G1 by APC/CCdh1 (indicated by dashed arrow) and in S phase by SCF ubiquitin ligase. See text for references.
Figure 2. Structure and regulation of Emi1 and Emi2/Erp1
(A) The Emi1 protein contains an F-box domain, a C-terminal Zn2+ binding region (ZBR), and a D-box. The role of the F-box is unclear, but the D-box is the domain through which Emi1 binds the D-box receptor on the core APC/C, whereas the ZBR domain seems to act to inhibit access of substrates to the complex. See text for details and references. Emi1 is a substrate of the E3 ubiquitin ligase SCFβ-TrCP (Guardavaccaro et al. 2003, Margottin-Goguet et al. 2003). Phosphorylation by both Cdk and Plk1 contribute to recognition and ubiquitination of Emi1 by SCFβ-TrCP in late prophase (Margottin-Goguet et al. 2003, Moshe et al. 2004, Hansen et al. 2004). (B) The Emi2/Erp1 protein also contains an F-box domain, a C-terminal Zn2+ binding region (ZBR), and a D-box. Upon fertilization, activated calmodulin kinase II (CaMKII) acts as a priming kinase for Plx1 on Emi2. Phosphorylation by Plx1 targets Emi2 for degradation targeted by SCFβ-TrCP. Phosphorylation of Emi2 by p90RSK, part of the pathway that establishes CSF arrest, is required for Emi2 stability during oocyte maturation. Phosphorylation of Emi2 by Cdc2 (Cdk1)/CycB may disrupt the interaction between Emi2 and APC/C. See text for details and references.
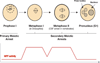
Figure 3. Meiotic Progression in Females
Oocytes are arrested in prophase I to allow for oocyte growth and differentiation before initiation of the meiotic divisions. Then, oocytes arrest again at different times to await egg activation or fertilization. This secondary arrest ensures that the completion of meiosis is properly coordinated with fertilization of the oocyte. In Drosophila, the secondary meiotic arrest takes place in metaphase I. Egg activation releases this arrest. In most vertebrates the secondary meiotic arrest takes place in metaphase II and is called cytostatic factor (CSF) arrest. CSF is released by fertilization. MPF (CycB/Cdc2 (Cdk1)) activity levels rise upon entry into the meiotic divisions. They must drop in between meiosis I and meiosis II to exit meiosis I, but some activity must be maintained to suppress DNA replication in between the two divisions. MPF levels drop again at exit from meiosis II. See text for details and references.
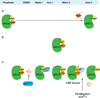
Figure 4. Meiotic APC/C Regulators
(A) In S. cerevisiae Mnd2 inhibits APC/CAma1, a meiosis-specific form of the APC/C, beginning in prophase. Inhibition is not released until late meiosis when Mnd2 protein disappears from meiotic cells. Full activation of APC/CAma1 may also require APC/CCdc20-mediated degradation of cyclins in meiosis I. (B) In S. pombe Mes1 is an inhibitor of APC/CSlp1/Cdc20 in between the meiotic divisions. Mes1 itself is an APC/C substrate but is able to compete for binding to the APC/C more efficiently than other substrates. (C) In vertebrates regulation of the APC/C during meiosis has been well-studied. In prophase, prior to germinal vesicle breakdown (GVBD), inhibition of APC/CCdh1 by Emi1 allows for an accumulation of MPF that is required for entry into the first meiotic division. Emi1 is targeted for degradation by SCFβ-TrCP at GVBD. APC/CCdc20 activity is thought to be regulated by the spindle assembly checkpoint in meiosis I for proper homolog alignment and disjunction. Similar to the role of Mes1, Emi2/Erp1 helps maintain low APC/CCdc20 activity levels in between the two meiotic divisions. Emi2/Erp1 is also a main component of CSF arrest and prevents APC/CCdc20 from being active until fertilization. The spindle assembly checkpoint may play a role is establishing but not in maintaining CSF arrest. At fertilization, a transient increase in free cytosolic Ca2+ activates calmodulin kinase II and the phosphatase calcineurin. Calmodulin kinase II phosphorylates Emi2, which ultimately leads to its degradation by SCFβ-TrCP. Calcineurin dephosphorylates Apc3, a core APC/C subunit, and Cdc20/Fzy, both of which may contribute to activation of APC/CCdc20 upon fertilization. See text for references and details.
Similar articles
- The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis.
Prinz S, Hwang ES, Visintin R, Amon A. Prinz S, et al. Curr Biol. 1998 Jun 18;8(13):750-60. doi: 10.1016/s0960-9822(98)70298-2. Curr Biol. 1998. PMID: 9651679 - Spindle checkpoint activation at meiosis I advances anaphase II onset via meiosis-specific APC/C regulation.
Yamamoto A, Kitamura K, Hihara D, Hirose Y, Katsuyama S, Hiraoka Y. Yamamoto A, et al. J Cell Biol. 2008 Jul 28;182(2):277-88. doi: 10.1083/jcb.200802053. Epub 2008 Jul 21. J Cell Biol. 2008. PMID: 18644893 Free PMC article. - Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint.
Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A. Yudkovsky Y, et al. Biochem Biophys Res Commun. 2000 May 10;271(2):299-304. doi: 10.1006/bbrc.2000.2622. Biochem Biophys Res Commun. 2000. PMID: 10799291 - The spindle checkpoint: how do cells delay anaphase onset?
Sczaniecka MM, Hardwick KG. Sczaniecka MM, et al. SEB Exp Biol Ser. 2008;59:243-56. SEB Exp Biol Ser. 2008. PMID: 18368927 Review. - Regulation of APC-Cdc20 by the spindle checkpoint.
Yu H. Yu H. Curr Opin Cell Biol. 2002 Dec;14(6):706-14. doi: 10.1016/s0955-0674(02)00382-4. Curr Opin Cell Biol. 2002. PMID: 12473343 Review.
Cited by
- Insights into APC/C: from cellular function to diseases and therapeutics.
Zhou Z, He M, Shah AA, Wan Y. Zhou Z, et al. Cell Div. 2016 Jul 13;11:9. doi: 10.1186/s13008-016-0021-6. eCollection 2016. Cell Div. 2016. PMID: 27418942 Free PMC article. Review. - Finishing the egg.
Berg C, Sieber M, Sun J. Berg C, et al. Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article. - Novel piperazine-based compounds inhibit microtubule dynamics and sensitize colon cancer cells to tumor necrosis factor-induced apoptosis.
Chopra A, Anderson A, Giardina C. Chopra A, et al. J Biol Chem. 2014 Jan 31;289(5):2978-91. doi: 10.1074/jbc.M113.499319. Epub 2013 Dec 12. J Biol Chem. 2014. PMID: 24338023 Free PMC article. - emb-1 encodes the APC16 subunit of the Caenorhabditis elegans anaphase-promoting complex.
Shakes DC, Allen AK, Albert KM, Golden A. Shakes DC, et al. Genetics. 2011 Oct;189(2):549-60. doi: 10.1534/genetics.111.131714. Epub 2011 Jul 20. Genetics. 2011. PMID: 21775471 Free PMC article. - Distinct requirements for the COMPASS core subunits Set1, Swd1, and Swd3 during meiosis in the budding yeast Saccharomyces cerevisiae.
Trainor BM, Ciccaglione K, Czymek M, Law MJ. Trainor BM, et al. G3 (Bethesda). 2021 Oct 19;11(11):jkab283. doi: 10.1093/g3journal/jkab283. G3 (Bethesda). 2021. PMID: 34849786 Free PMC article.
References
- Asakawa H, Kitamura K, Shimoda C. A novel Cdc20-related WD-repeat protein, Fzr1, is required for spore formation in Schizosaccharomyces pombe. Mol Genet Genomics. 2001;265:424–435. - PubMed
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. - PubMed
- Ban KH, Torres JZ, Miller JJ, Mikhailov A, Nachury MV, et al. The END network couples spindle pole assembly to inhibition of the anaphase-promoting complex/cyclosome in early mitosis. Dev Cell. 2007;13:29–42. - PubMed
- Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle. 2005;4:1230–1232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous