Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? - PubMed (original) (raw)
doi: 10.1186/1479-5876-6-35.
Carlos E Ambrosio, Alexandre Kerkis, Daniele S Martins, Eder Zucconi, Simone A S Fonseca, Rosa M Cabral, Carlos M C Maranduba, Thais P Gaiad, Adriana C Morini, Natassia M Vieira, Marina P Brolio, Osvaldo A Sant'Anna, Maria A Miglino, Mayana Zatz
Affiliations
- PMID: 18598348
- PMCID: PMC2529267
- DOI: 10.1186/1479-5876-6-35
Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic?
Irina Kerkis et al. J Transl Med. 2008.
Abstract
Background: The golden retriever muscular dystrophy (GRMD) dogs represent the best available animal model for therapeutic trials aiming at the future treatment of human Duchenne muscular dystrophy (DMD). We have obtained a rare litter of six GRMD dogs (3 males and 3 females) born from an affected male and a carrier female which were submitted to a therapeutic trial with adult human stem cells to investigate their capacity to engraft into dogs muscles by local as compared to systemic injection without any immunosuppression.
Methods: Human Immature Dental Pulp Stem Cells (hIDPSC) were transplanted into 4 littermate dogs aged 28 to 40 days by either arterial or muscular injections. Two non-injected dogs were kept as controls. Clinical translation effects were analyzed since immune reactions by blood exams and physical scores capacity of each dog. Samples from biopsies were checked by immunohistochemistry (dystrophin markers) and FISH for human probes.
Results and discussion: We analyzed the cells' ability in respect to migrate, engraftment, and myogenic potential, and the expression of human dystrophin in affected muscles. Additionally, the efficiency of single and consecutive early transplantation was compared. Chimeric muscle fibers were detected by immunofluorescence and fluorescent in situ hybridisation (FISH) using human antibodies and X and Y DNA probes. No signs of immune rejection were observed and these results suggested that hIDPSC cell transplantation may be done without immunosuppression. We showed that hIDPSC presented significant engraftment in GRMD dog muscles, although human dystrophin expression was modest and limited to several muscle fibers. Better clinical condition was also observed in the dog, which received monthly arterial injections and is still clinically stable at 25 months of age.
Conclusion: Our data suggested that systemic multiple deliveries seemed more effective than local injections. These findings open important avenues for further researches.
Figures
Figure 1
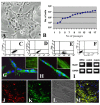
Characterization, myogenic differentiation in vitro and migrating capacity in vivo of hIDPSC. (A) Fibroblast-like morphology of hIDPSC. (B) Proliferating potential of these cells during 16 successive passages. (C-E) Flow cytometry showing expression of CD105 (SH2), CD73 (SH3 and SH4), respectively. (F) Negative control for respective isotype. (G,H) Myogenic differentiation in vitro: fused hIDPSC forming myotubes. Positive immunostaining with α-actinin (G) and myosin (H): insets in (G and H) show details of anti-bodies localization within myotubes, higher magnification. (I) RT-PCR analysis of the expression of human dystrophin (MyoD1) observed hIDPSC and human muscles, used as a positive control. (J-M). Migrating capacity of hIDPSC visualized 30 days after their intraperitoneal injection into normal mice (J-M): (J) Cells stained with DiI-Vybrant (red) in mouse, (K) positive reaction with primary human anti-IDPSC antibody in mice (secondary antibody FITC-conjugated was used (green), (L) Morphology of mouse cardiac muscles. (M) merged image of J-L. A= light microscopy, phase contrast, G-H = epi-fluorescence (EF), J-M= confocal microscopy: J, K = fluorescent microscopy (Fcm), L = DIC (Differential interference contrast) M = Fcm+DIC. Scale bars: G,H = 10 μm, A,J-M = 100 μm, N-P = 50 μm
Figure 2
DMD genotyping. GRMD puppies from a colony of dogs with X-linked muscular dystrophy were genotyped within 48 hs after birth. The genomic PCR product digested with Sau96I produces the wild type band (310 pb) and the mutant band (150 pb) labelled with arrows. ■ = Affected male. ● = Affected female. ◉ = Carrier female.
Figure 3
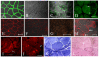
Representative figures of hIDPSC engraftment observed within canine skeletal muscles (GRMD). (A) FT2-IM, after 107 days of single transplantation: Positive immunostaining with anti-hIDPSC antibody (green) was observed in several muscle fibers (white arrowhead) and in the nuclei (white arrows, blue, DAPI stained superposition with anti-IDPSC antibody, green) of hIDPSC localized in the periphery of canine muscle fibers. (B-I) One year after multiple hIDPSC transplantation. Positive immunostaining with anti-IDPSC antibody was observed in MT1-S muscles: (B-D) transversal and (E-H) longitudinal sections. Inset in (F) demonstrate (higher magnification) skeletal muscle Z-bands (red arrowhead) observed in the local of positive immunostaining with anti-hIDPSC antibody (DIC). (H) Higher magnification of (G) demonstrating positive reaction of hIDPSC antibody (green) with skeletal muscle Z-bands (red arrowhead). (I) Chimeric human/canine muscle fiber only a half of which presents positive green fluorescent immunostaining (green). (J) Control: affected male without hIDPSC transplantation immunostained with anti-hIDPSC antibody did not present any labeling. (K) FISH analysis of dystrophic male's muscles using specific human probe for chromosome: Y (red) and in inset X (yellow, as a result of merged images of PI (red) stained nucleus and probe of chromosome X (green) are presented). (L-P) Immunostaining using FITC conjugated anti-human nucleus (anti-HN) antibody (green). (L) Positive control. Merged image of positive staining with anti-HN antibody and nuclei stained with DAPI in normal human muscles. (M-O) Positive immunostaining with anti-HN antibody observed in the nuclei of hIDPSC (green) engrafted into canine muscle fibers of MT1-S. They (white arrowhead) can be seen within canine muscle fibers and in perimysium (white arrows). Canine nuclei (group of 4 nuclei indicated by red arrow) did not present any reaction with anti-HN antibody. (P) Negative control. Muscles of normal dog did not react with anti-HN antibody. Only nuclei stained with DAPI can be observed. Confocal microscopy, A,D,G-L,P = Fcm+DIC; C,F,M,N = Fcm, B,E, Inset in (F) = DIC, Scale bars: A-H, L,P = 50 μm, K = 100 μm, and M-O = 20 μm, Inset in (K) = 10 μm
Figure 4
Immunofluorescence analysis using the specific human anti-dystrophin monoclonal antibodies: Mandys106 2C6 (A-D) and C-terminal (E-J) antibodies one year after the hIDPSC transplantation. (A) Positive control: expression of Mandys106 2C6 antibody in normal human muscles. (B) Negative control: lack of expression of same antibody in the muscles of normal dog. (C, D) MT1-S shows positive staining with Mandys106 2C6 in large dystrophic fibers (white arrows). (E) Positive control: expression of C-terminal in normal human muscles. (F) C-terminal antibody in the muscles of normal dog presents weak labeling. (G) Negative control: lack of its expression in the muscles of affected dog. (H-J) MT1-S shows positive staining with C-terminal antibody in some large dystrophic fibers (arrow). K) Toluidine blue and L) HE staining shows very large fibers with multiple centrally located nuclei and splitting. A, C, E-H = Fcm + DIC, B = DIC, D,I,= EF, J,K = Light microscopy, Scale bars = 50 μm
Similar articles
- Identification in GRMD dog muscle of critical miRNAs involved in pathophysiology and effects associated with MuStem cell transplantation.
Robriquet F, Babarit C, Larcher T, Dubreil L, Ledevin M, Goubin H, Rouger K, Guével L. Robriquet F, et al. BMC Musculoskelet Disord. 2016 May 11;17:209. doi: 10.1186/s12891-016-1060-5. BMC Musculoskelet Disord. 2016. PMID: 27170302 Free PMC article. - Human adipose-derived mesenchymal stromal cells injected systemically into GRMD dogs without immunosuppression are able to reach the host muscle and express human dystrophin.
Vieira NM, Valadares M, Zucconi E, Secco M, Bueno CR Jr, Brandalise V, Assoni A, Gomes J, Landini V, Andrade T, Caetano HV, Vainzof M, Zatz M. Vieira NM, et al. Cell Transplant. 2012;21(7):1407-17. doi: 10.3727/096368911X. Cell Transplant. 2012. PMID: 23168016 - Differential Gene Expression Profiling of Dystrophic Dog Muscle after MuStem Cell Transplantation.
Robriquet F, Lardenois A, Babarit C, Larcher T, Dubreil L, Leroux I, Zuber C, Ledevin M, Deschamps JY, Fromes Y, Cherel Y, Guevel L, Rouger K. Robriquet F, et al. PLoS One. 2015 May 8;10(5):e0123336. doi: 10.1371/journal.pone.0123336. eCollection 2015. PLoS One. 2015. PMID: 25955839 Free PMC article. - The golden retriever model of Duchenne muscular dystrophy.
Kornegay JN. Kornegay JN. Skelet Muscle. 2017 May 19;7(1):9. doi: 10.1186/s13395-017-0124-z. Skelet Muscle. 2017. PMID: 28526070 Free PMC article. Review. - Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies.
Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Li J, Nghiem P, Detwiler DA, Larsen CA, Grange RW, Bhavaraju-Sanka RK, Tou S, Keene BP, Howard JF Jr, Wang J, Fan Z, Schatzberg SJ, Styner MA, Flanigan KM, Xiao X, Hoffman EP. Kornegay JN, et al. Mamm Genome. 2012 Feb;23(1-2):85-108. doi: 10.1007/s00335-011-9382-y. Epub 2012 Jan 5. Mamm Genome. 2012. PMID: 22218699 Free PMC article. Review.
Cited by
- Redefining the potential applications of dental stem cells: An asset for future.
Rai S, Kaur M, Kaur S, Arora SP. Rai S, et al. Indian J Hum Genet. 2012 Sep;18(3):276-84. doi: 10.4103/0971-6866.107976. Indian J Hum Genet. 2012. PMID: 23716933 Free PMC article. - Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods.
Karamzadeh R, Eslaminejad MB, Aflatoonian R. Karamzadeh R, et al. J Vis Exp. 2012 Nov 24;(69):4372. doi: 10.3791/4372. J Vis Exp. 2012. PMID: 23208006 Free PMC article. - Motor physical therapy affects muscle collagen type I and decreases gait speed in dystrophin-deficient dogs.
Gaiad TP, Araujo KP, Serrão JC, Miglino MA, Ambrósio CE. Gaiad TP, et al. PLoS One. 2014 Apr 8;9(4):e93500. doi: 10.1371/journal.pone.0093500. eCollection 2014. PLoS One. 2014. PMID: 24713872 Free PMC article. - Profiles of Steroid Hormones in Canine X-Linked Muscular Dystrophy via Stable Isotope Dilution LC-MS/MS.
Martins-Júnior HA, Simas RC, Brolio MP, Ferreira CR, Perecin F, Nogueira Gde P, Miglino MA, Martins DS, Eberlin MN, Ambrósio CE. Martins-Júnior HA, et al. PLoS One. 2015 May 26;10(5):e0126585. doi: 10.1371/journal.pone.0126585. eCollection 2015. PLoS One. 2015. PMID: 26010907 Free PMC article. - Dental Pulp Stem Cells: Current Advances in Isolation, Expansion and Preservation.
Rodas-Junco BA, Villicaña C. Rodas-Junco BA, et al. Tissue Eng Regen Med. 2017 Mar 14;14(4):333-347. doi: 10.1007/s13770-017-0036-3. eCollection 2017 Aug. Tissue Eng Regen Med. 2017. PMID: 30603490 Free PMC article. Review.
References
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources