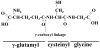Regulation of glutathione synthesis - PubMed (original) (raw)
Review
Regulation of glutathione synthesis
Shelly C Lu. Mol Aspects Med. 2009 Feb-Apr.
Abstract
Glutathione (GSH) is a ubiquitous intracellular peptide with diverse functions that include detoxification, antioxidant defense, maintenance of thiol status, and modulation of cell proliferation. GSH is synthesized in the cytosol of all mammalian cells in a tightly regulated manner. The major determinants of GSH synthesis are the availability of cysteine, the sulfur amino acid precursor, and the activity of the rate-limiting enzyme, glutamate cysteine ligase (GCL). GCL is composed for a catalytic (GCLC) and modifier (GCLM) subunit and they are regulated at multiple levels and at times differentially. The second enzyme of GSH synthesis, GSH synthase (GS) is also regulated in a coordinated manner as GCL subunits and its up-regulation can further enhance the capacity of the cell to synthesize GSH. Oxidative stress is well known to induce the expression of GSH synthetic enzymes. Key transcription factors identified thus far include Nrf2/Nrf1 via the antioxidant response element (ARE), activator protein-1 (AP-1) and nuclear factor kappa B (NFkappaB). Dysregulation of GSH synthesis is increasingly being recognized as contributing to the pathogenesis of many pathological conditions. These include diabetes mellitus, pulmonary fibrosis, cholestatic liver injury, endotoxemia and drug-resistant tumor cells. Manipulation of the GSH synthetic capacity is an important target in the treatment of many of these disorders.
Figures
Fig. 1
Structure of GSH or γ-glutamylcysteinyl glycine, where the N-terminal glutamate and cysteine are linked by the γ-carboxyl group of glutamate.
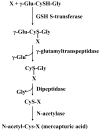
Fig. 2
Detoxifying action of GSH through the mercapturic pathway. X is a compound with an electrophilic center that can form GSH conjugate in a reaction catalyzed by GSH S-transferase. The γ-glutamyl moiety is then cleaved by γ-glutamyltranspeptidase, releasing the cysteinyl-glycine conjugate. This is further broken down by dipeptidase, resulting in the formation of the cysteinyl conjugate. This is followed by N-acetylation of the cysteine conjugate catalyzed by N-acetylase, forming a mercapturic acid.
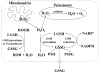
Fig. 3
GSH is an important antioxidant. Hydrogen peroxide, generated as a result of aerobic metabolism, can be metabolized by GSH peroxidase in the cytosol and mitochondria, and by catalase in the peroxisome. GSSG that is formed is reduced back to GSH by GSSG reductase at the expense of NADPH, thereby forming a redox cycle. Organic peroxides (ROOH) can be reduced by either GSH peroxidase or GSH S-transferase. Under severe oxidative stress, the ability of the cell to reduce GSSG to GSH may be overcome, leading to an accumulation of GSSG. To avoid a shift in the redox equilibrium, GSSG can either be actively transported out of the cell or react with a protein sulfhydryl (PSH) to form a mixed disulfide (PSSG).
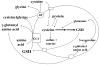
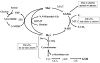
Fig. 4
GSH as cysteine storage via the γ-glutamyl cycle. The γ-glutamyl cycle utilize GSH as a continuous source of cysteine. Cysteine is taken up readily by most cells and once it enters the cell, most of it is incorporated into GSH while the rest is incorporated into newly synthesized proteins and/or broken down into sulfate and taurine. GSH is exported from the cell and the ecto-enzyme GGT then transfers the γ-glutamyl moiety of GSH to an amino acid (the best acceptor being cystine), forming γ-glutamyl amino acid and cysteinylglycine. The γ-glutamyl amino acid can then be transported back into the cell to complete the cycle. Once inside the cell, the γ-glutamyl amino acid can be further metabolized to release the amino acid and 5-oxoproline, which can be converted to glutamate. Cysteinylglycine is broken down by dipeptidase (DP) to generate cysteine and glycine, which are then transported back into the cell to be reincorporated into GSH.
Fig. 5
Hepatic methionine metabolism and GSH synthesis. Up to half of the daily intake of methionine (Met) is catabolized to S-adenosylmethionine (SAMe) in the liver in a reaction catalyzed by methionine adenosyltransferase (MAT). SAMe is the link to three key metabolic pathways - polyamine synthesis, transmethylation and transsulfuration. Polyamine synthesis is required for cell growth and here SAMe is decarboxylated and the remaining propylamino moiety is donated to putrescine and spermidine. In transmethylation, SAMe donates its methyl group to a large variety of acceptor molecules in reactions catalyzed by methyltransferases (MTs). S-adenosylhomocysteine (SAH), generated as a result of transmethylation, is a potent inhibitor of all transmethylation reactions. Hydrolysis of SAH to homocysteine (Hcy) and adenosine is through a reversible reaction catalyzed by SAH hydrolase, whose thermodynamics favors biosynthesis rather than hydrolysis. In vivo this reaction proceeds as hydrolysis because the products Hcy and adenosine are promptly removed. Hcy can be remethylated to form methionine via methionine synthase (MS), which requires folate and vitamin B12 and betaine homocysteine methyltransferase (BHMT), which requires betaine. MS-mediated homocysteine remethylation requires 5-methyltetrahydrofolate (5-MTHF), which is generated from 5,10-methylenetetrahydrofolate (5,10-MTHF) in a reaction catalyzed by methylenetetrahydrofolate reductase. 5-MTHF is then converted to tetrahydrofolate (THF) as it donates its methyl group and THF is converted back to 5,10-MTHF. In trans-sulfuration, Hcy is converted to cysteine (Cys), the rate-limiting precursor for GSH, via a two-step enzymatic process catalyzed by cystathionine β-synthase (CBS) and cystathionase, both requiring vitamin B6. Cys is then converted to GSH. The trans-sulfuration pathway is particularly active in the liver and allows methionine and SAMe to be effectively utilized as GSH precursor.
Similar articles
- Glutathione synthesis.
Lu SC. Lu SC. Biochim Biophys Acta. 2013 May;1830(5):3143-53. doi: 10.1016/j.bbagen.2012.09.008. Epub 2012 Sep 17. Biochim Biophys Acta. 2013. PMID: 22995213 Free PMC article. Review. - Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1.
Yang H, Magilnick N, Ou X, Lu SC. Yang H, et al. Biochem J. 2005 Oct 15;391(Pt 2):399-408. doi: 10.1042/BJ20050795. Biochem J. 2005. PMID: 16011481 Free PMC article. - Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: alterations in the activities and protein expression of glutathione-related enzymes.
Gu F, Chauhan V, Chauhan A. Gu F, et al. Free Radic Biol Med. 2013 Dec;65:488-496. doi: 10.1016/j.freeradbiomed.2013.07.021. Epub 2013 Jul 26. Free Radic Biol Med. 2013. PMID: 23892356 - Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase.
Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Franklin CC, et al. Mol Aspects Med. 2009 Feb-Apr;30(1-2):86-98. doi: 10.1016/j.mam.2008.08.009. Epub 2008 Sep 6. Mol Aspects Med. 2009. PMID: 18812186 Free PMC article. Review. - Manipulation of cellular GSH biosynthetic capacity via TAT-mediated protein transduction of wild-type or a dominant-negative mutant of glutamate cysteine ligase alters cell sensitivity to oxidant-induced cytotoxicity.
Backos DS, Brocker CN, Franklin CC. Backos DS, et al. Toxicol Appl Pharmacol. 2010 Feb 15;243(1):35-45. doi: 10.1016/j.taap.2009.11.010. Epub 2009 Nov 13. Toxicol Appl Pharmacol. 2010. PMID: 19914271 Free PMC article.
Cited by
- GC-MS Studies on the Conversion and Derivatization of γ-Glutamyl Peptides to Pyroglutamate (5-Oxo-Proline) Methyl Ester Pentafluoropropione Amide Derivatives.
Bollenbach A, Tsikas D. Bollenbach A, et al. Molecules. 2022 Sep 15;27(18):6020. doi: 10.3390/molecules27186020. Molecules. 2022. PMID: 36144754 Free PMC article. - ALKBH5-mediated CHAC1 depletion promotes malignant progression and decreases cisplatin-induced oxidative stress in gastric cancer.
Chen C, Zhai E, Liu Y, Qian Y, Zhao R, Ma Y, Liu J, Huang Z, Chen J, Cai S. Chen C, et al. Cancer Cell Int. 2023 Nov 25;23(1):293. doi: 10.1186/s12935-023-03129-9. Cancer Cell Int. 2023. PMID: 38007439 Free PMC article. - System Xc -/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy.
Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG, Gao LC. Li FJ, et al. Front Pharmacol. 2022 Aug 29;13:910292. doi: 10.3389/fphar.2022.910292. eCollection 2022. Front Pharmacol. 2022. PMID: 36105219 Free PMC article. Review. - Evidence-based pathogenesis and treatment of ulcerative colitis: A causal role for colonic epithelial hydrogen peroxide.
Pravda J. Pravda J. World J Gastroenterol. 2022 Aug 21;28(31):4263-4298. doi: 10.3748/wjg.v28.i31.4263. World J Gastroenterol. 2022. PMID: 36159014 Free PMC article. Review. - Significance and Applications of the Thermo-Acidophilic Microalga Galdieria sulphuraria (Cyanidiophytina, Rhodophyta).
Retta B, Iovinella M, Ciniglia C. Retta B, et al. Plants (Basel). 2024 Jun 27;13(13):1786. doi: 10.3390/plants13131786. Plants (Basel). 2024. PMID: 38999626 Free PMC article. Review.
References
- Akerboom TP, Bilizer MM, Sies H. The relationship of biliary GSSG efflux and intracellular GSSG content in perfused rat liver. J Biol Chem. 1982;257:4248–4252. - PubMed
- Alhamdani MSS. Impairment of glutathione biosynthetic pathway in uremia and dialysis. Nephrol Dial Transplant. 2005;20:124–128. - PubMed
- Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membrane Biol. 1986;89:1–8. - PubMed
- Benassi B, Fanciulli M, Fiorentino F, Porrello A, Chiorino G, Loda M, Zupi G, Biroccio A. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell. 2006;21:509–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK045334/DK/NIDDK NIH HHS/United States
- R01 DK045334-15/DK/NIDDK NIH HHS/United States
- R56 DK045334/DK/NIDDK NIH HHS/United States
- DK45334/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous