Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice - PubMed (original) (raw)
Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice
J L Spencer et al. Neuroscience. 2008.
Erratum in
- Neuroscience. 2009 Sep 15;162(4):1437
Abstract
Estradiol modulates dendritic spine morphology and synaptic protein expression in the rodent hippocampus, as well as hippocampal-dependent learning and memory. In the rat, these effects may be mediated through nongenomic steroid signaling such as estradiol activation of the Akt and LIM kinase (LIMK) pathways, in addition to genomic signaling involving estradiol upregulation of brain-derived neurotrophic factor expression (BDNF). Due to the many species differences between mice and rats, including differences in the hippocampal response to estradiol, it is unclear whether estradiol modulates these pathways in the mouse hippocampus. Therefore, we investigated whether endogenous fluctuations of gonadal steroids modulate hippocampal activation of the Akt, LIMK, and the BDNF receptor TrkB in conjunction with spatial memory in female C57BL/6 mice. We found that Akt, LIMK, and TrkB were activated throughout the dorsal hippocampal formation during the high-estradiol phase, proestrus. Cycle phase also modulated expression of the pre- and post-synaptic markers synaptophysin and post-synaptic density 95. However, cycle phase did not influence performance on an object placement test of spatial memory, although this task is known to be sensitive to the complete absence of ovarian hormones. The findings suggest that endogenous estradiol and progesterone produced by the ovaries modulate specific signaling pathways governing actin remodeling, cell excitability, and synapse formation.
Figures
Figure 1
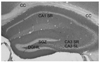
Density of peroxidase labeling was quantified in subregions of the dorsal hippocampal formation at −1.28 mm from bregma, shown on this section of dorsal hippocampus labeled for synaptophysin. Hippocampal subregions were chosen based on the anatomy of neuronal projections, previously demonstrated hormone sensitivity, and ease of identification in labeled sections. Additional measurements were taken from the sensory cortex overlying the dorsal hippocampus (not shown). All measurements were normalized to background measurements taken from the corpus callosum in the same image. CA1 SR, CA1 stratum radiatum; CA3 SL, CA3 stratum lucidum; CA3 SR, CA3 stratum radiatum; DGHIL, hilus of the dentate gyrus; SGZ, subgranular zone (pTrkB only); CC, corpus callosum. For all subsequent quantitative immunohistochemistry, n = 4 for proestrus, 6 for estrus, and 11 for diestrus.
Figure 2
The C57BL6 female mice used in this study had regular, 6.1-day estrous cycles. As assessed by daily morning vaginal smears, 1.1 days (18%) of the cycle were spent in proestrus, 2.5 days (41%) in estrus, and 2.5 days (41%) in diestrus. Mice were perfused as indicated with arrows in proestrus, early estrus, and early diestrus. Error bars show standard error of the mean.
Figure 3
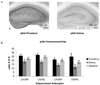
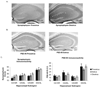
Akt is activated during proestrus and inactivated during estrus. A, Images of peroxidase labeling of phosphorylated Akt in the dorsal hippocampal formation of representative sections from one proestrus and one estrus mouse. pAkt immunoreactivity (IR) is darker throughout the hippocampus in proestrus than in estrus. B, quantification of pAkt-IR in four hippocampal subregions across the estrus cycle. pAkt=IR was significantly higher in proestrus compared to estrus (p<0.0001), and in diestrus compared to estrus (p=0.0001). R.O.D., relative optical density.
Figure 4
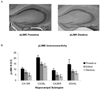
LIMK is activated during proestrus. A, Images of peroxidase labeling of phosphorylated LIMK in the dorsal hippocampal formation of representative sections from one proestrus and one diestrus mouse. pLIMK IR is darker throughout the hippocampus in proestrus than in diestrus. B, quantification of pLIMK-IR in four hippocampal subregions across the estrous cycle. pLIMK-IR was significantly higher in proestrus than in estrus (p<0.01) or diestrus (p<0.0001).
Figure 5
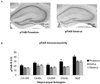
The neurotrophin receptor TrkB is activated during proestrus. A, Images of peroxidase labeling of phosphorylated TrkB in the dorsal hippocampal formation of representative sections from one proestrus and one diestrus mouse. pTrkB IR is darker throughout the hippocampus in proestrus than in diestrus. B, quantification of pTrkB-IR in five hippocampal subregions. pTrkB-IR was significantly higher in proestrus compared to estrus and diestrus (p<0.0001).
Figure 6
Expression of the presynaptic protein synaptophysin and the postsynaptic protein PSD-95 fluctuates across the mouse estrous cycle. A and B, representative images from the dorsal hippocampal formation of synaptophysin and PSD-95 IR in mice at different stages of the estrous cycle. C, quantification of synaptophysin and PSD-95 IR in four hippocampal subregions. Synaptophysin IR was significantly higher in diestrus than in proestrus (p<0.05). In contrast, PSD-95 IR was higher in proestrus than in estrus (p<0.05) or diestrus (p<0.01).
Figure 7
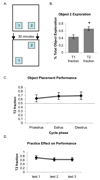
Performance of female mice on an object placement test of 30-minute spatial memory retention is not affected by estrous cycle phase. A, Schematic diagram shows the position of objects during the sample trial (T1) and recognition trial 30 minutes later (T2), with object 2 in a new location. B, Mice explored the object in a novel location significantly more than chance during the recognition trial. The graphs show amount of time spent exploring object 2 during the sample (T1) trial and recognition (T2) trial as a percentage of total time spent exploring both objects during each trial. *p<0.005 relative to chance (0.5). C, Performance on the task was not affected by cycle phase. The graph shows amount of time spent exploring object 2 during the recognition (T2) trials as a percentage of total object exploration time during proestrus, estrus and diestrus. D, Performance was not affected by repeated testing. Graph shows total exploration time during the (T1) trial for the first, second and third tests conducted. No significant differences were found between groups. n=10 mice, each tested in proestrus, estrus, and diestrus.
Figure 8
Summary diagram shows levels of pAkt-, pLIMK-, pTrkB-, synaptophysin-, and PSD-95-IR as well as spatial memory across the estrous cycle of the mouse in comparison to known fluctuations of circulating estradiol and progesterone.
Similar articles
- Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation.
Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Spencer-Segal JL, et al. Neuroscience. 2012 Jan 27;202:131-46. doi: 10.1016/j.neuroscience.2011.11.035. Epub 2011 Nov 23. Neuroscience. 2012. PMID: 22133892 Free PMC article. - Antagonism of brain insulin-like growth factor-1 receptors blocks estradiol effects on memory and levels of hippocampal synaptic proteins in ovariectomized rats.
Nelson BS, Springer RC, Daniel JM. Nelson BS, et al. Psychopharmacology (Berl). 2014 Mar;231(5):899-907. doi: 10.1007/s00213-013-3310-7. Epub 2013 Oct 22. Psychopharmacology (Berl). 2014. PMID: 24146138 Free PMC article. - TrkB phosphorylation by Cdk5 is required for activity-dependent structural plasticity and spatial memory.
Lai KO, Wong AS, Cheung MC, Xu P, Liang Z, Lok KC, Xie H, Palko ME, Yung WH, Tessarollo L, Cheung ZH, Ip NY. Lai KO, et al. Nat Neurosci. 2012 Nov;15(11):1506-15. doi: 10.1038/nn.3237. Epub 2012 Oct 14. Nat Neurosci. 2012. PMID: 23064382 Free PMC article. - Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice.
Ma J, Zhang Z, Kang L, Geng D, Wang Y, Wang M, Cui H. Ma J, et al. Exp Gerontol. 2014 Oct;58:256-68. doi: 10.1016/j.exger.2014.08.011. Epub 2014 Aug 27. Exp Gerontol. 2014. PMID: 25172625 - Regulation of object recognition and object placement by ovarian sex steroid hormones.
Tuscher JJ, Fortress AM, Kim J, Frick KM. Tuscher JJ, et al. Behav Brain Res. 2015 May 15;285:140-57. doi: 10.1016/j.bbr.2014.08.001. Epub 2014 Aug 15. Behav Brain Res. 2015. PMID: 25131507 Free PMC article. Review.
Cited by
- Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse.
Hara Y, Waters EM, McEwen BS, Morrison JH. Hara Y, et al. Physiol Rev. 2015 Jul;95(3):785-807. doi: 10.1152/physrev.00036.2014. Physiol Rev. 2015. PMID: 26109339 Free PMC article. Review. - Distinct Antidepressant-Like and Cognitive Effects of Antidepressants with Different Mechanisms of Action in Middle-Aged Female Mice.
Li Y, Sanchez C, Gulinello M. Li Y, et al. Int J Neuropsychopharmacol. 2017 Jun 1;20(6):510-515. doi: 10.1093/ijnp/pyx004. Int J Neuropsychopharmacol. 2017. PMID: 28158336 Free PMC article. - LIM-Kinases in Synaptic Plasticity, Memory, and Brain Diseases.
Ben Zablah Y, Zhang H, Gugustea R, Jia Z. Ben Zablah Y, et al. Cells. 2021 Aug 13;10(8):2079. doi: 10.3390/cells10082079. Cells. 2021. PMID: 34440848 Free PMC article. Review. - Estrous cycle-dependent neurovascular dysfunction induced by angiotensin II in the mouse neocortex.
Capone C, Anrather J, Milner TA, Iadecola C. Capone C, et al. Hypertension. 2009 Aug;54(2):302-7. doi: 10.1161/HYPERTENSIONAHA.109.133249. Epub 2009 Jun 8. Hypertension. 2009. PMID: 19506098 Free PMC article. - Sex-specific features of spine densities in the hippocampus.
Brandt N, Löffler T, Fester L, Rune GM. Brandt N, et al. Sci Rep. 2020 Jul 9;10(1):11405. doi: 10.1038/s41598-020-68371-x. Sci Rep. 2020. PMID: 32647191 Free PMC article.
References
- Aloysi A, Van Dyk K, Sano M. Women's cognitive and affective health and neuropsychiatry. Mt Sinai J Med. 2006;73:967–975. - PubMed
- Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. - PubMed
- Ballare C, Vallejo G, Vicent GP, Saragueta P, Beato M. Progesterone signaling in breast and endometrium. J Steroid Biochem Mol Biol. 2006;102:2–10. - PubMed
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS007080/NS/NINDS NIH HHS/United States
- GM07739/GM/NIGMS NIH HHS/United States
- NS007080/NS/NINDS NIH HHS/United States
- R01 DA008259/DA/NIDA NIH HHS/United States
- MH082528/MH/NIMH NIH HHS/United States
- F30 MH082528/MH/NIMH NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- DA08259/DA/NIDA NIH HHS/United States
- HL18974/HL/NHLBI NIH HHS/United States
- F32 DK117510/DK/NIDDK NIH HHS/United States
- P01 HL018974/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases