Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization - PubMed (original) (raw)
Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization
Veit Hornung et al. Nat Immunol. 2008 Aug.
Abstract
Inhalation of silica crystals causes inflammation in the alveolar space. Prolonged exposure to silica can lead to the development of silicosis, an irreversible, fibrotic pulmonary disease. The mechanisms by which silica and other crystals activate immune cells are not well understood. Here we demonstrate that silica and aluminum salt crystals activated inflammasomes formed by the cytoplasmic receptor NALP3. NALP3 activation required phagocytosis of crystals, and this uptake subsequently led to lysosomal damage and rupture. 'Sterile' lysosomal damage (without crystals) also induced NALP3 activation, and inhibition of either phagosomal acidification or cathepsin B activity impaired NALP3 activation. Our results indicate that the NALP3 inflammasome senses lysosomal damage as an endogenous 'danger' signal.
Figures
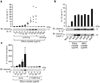
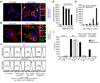
Fig. 1. Silica induces release of mature IL-1β and activated caspase-1 in human PBMCs in a caspase-1 dependent manner
(a) Human PBMCs were primed with LPS (25 pg/ml) or left untreated for 3h and subsequently stimulated with silica crystals or controls. After 6h, supernatants were assessed for IL-1β production by ELISA and Western blot. ELISA data of four independent donors are depicted (upper panel) and Western blot analysis of one representative donor is shown (lower panel). (b) LPS-primed human PBMCs were stimulated with either silica crystals, MSU crystals, ATP or transfected with dAdT. 6h after stimulation, supernatants were analyzed for IL-1β by ELISA and assessed for matured IL-1β or activated caspase-1 by Western blot. Data of one representative donor out of three are depicted. (c) Human LPS-primed PBMCs were stimulated with silica crystals in the presence or absence of the caspase-1 inhibitor z-YVAD (10 µM). After 6h, supernatants were assessed for IL-1β by ELISA and Western blot. Mean values (+SD) of two donors are depicted as fold increase (ELISA) and Western blot data of one representative donor are shown.
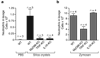
Fig. 2. Silica-mediated neutrophil influx in a model of acute lung inflammation is mediated by IL-1
(a) Silica crystals (200 µg / mouse) were orotracheally instilled into wild-type mice, MyD88/TRIF-double-deficient mice or IL-1R-deficient mice. 16–18h after instillation, neutrophil counts were monitored in the lung lavage by FACS. (b) In addition, wild-type mice, MyD88-/TRIF-double-deficient mice or IL-1R-deficient mice were orotracheally challenged with zymosan (50 µg / mouse) and processed as in (a).
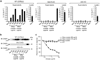
Fig. 3. Silica-mediated release of matured IL-1β and activated caspase-1 is mediated by the NALP3 inflammasome
(a) Bone marrow-derived macrophages of wild-type mice, NALP3-deficient mice or ASC-deficient mice were primed with LPS for 3h and subsequently stimulated with either silica crystals, MSU crystals, ATP or transfected with dAdT. 6h after stimulation, supernatants were analyzed for IL-1β by ELISA (supernatants). To assess intracellular pro-IL-1β, cell lysates from primed, but unstimulated macrophages were assessed for IL-1β by ELISA (lysate). (b) In addition, supernatants were assessed for activated caspase-1 by Western blot. Data from one representative experiment out of two are depicted. (c) LPS-primed mouse macrophages were stimulated with silica or MSU crystals as indicated in absence or presence of uricase as shown. Supernatants were assessed for IL-1β by ELISA.
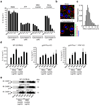
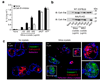
Fig. 4. Crystal uptake is required for inflammasome activation, whereas the phagosomal ROS system is not involved in crystal-mediated inflammasome activation
(a) Human LPS-primed PBMCs were treated with cytochalasin D in ascending doses and subsequently stimulated with silica crystals, MSU crystals or ATP. IL-1β release was measured by ELISA 6h after stimulation. Data from one representative experiment out of two are depicted. (b) B6-MCLs were stimulated for 2h with silica crystals in the presence or absence of cytochalasin D (2.5 µM). Cells were then membrane stained with fluorescent choleratoxin (red), nuclei stained with Hoechst dye (blue) and analyzed for crystal uptake (green) using confocal microscopy. (c) B6-MCLs were incubated with silica crystals as in (b) and phagocytosed silica crystals was analyzed for their length and the fractional distribution of crystal sizes is shown from phagocytosed crystals of 10 representative cells. (d and e) Bone marrow-derived macrophages of wild-type mice, gp91Phox-deficient mice or IPAF-deficient mice (as a mixed background control) were primed with LPS for 3h and subsequently stimulated with either silica crystals, MSU crystals, ATP or transfected with dAdT. 6h after stimulation, supernatants were analyzed for IL-1β by ELISA (d) and assessed for activated caspase-1 by Western blot (e). Data from one representative experiment out of two are depicted.
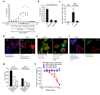
Fig. 5. Phagocytosis of crystals leads to lysosomal destabilization
(a) B6-MCLs were incubated with 10 µg/ml DQ-ovalbumin (red) alone or together with silica crystals (green) for 60 min, surface stained with fluorescent choleratoxin (blue) and analyzed by confocal microscopy. (b) B6-MCLs were incubated with silica crystals (green) for 60 min or left untreated, fixed, permeabilized (saponin 0.01%) and stained with fluorescent choleratoxin (red), Hoechst dye (blue) and analyzed by confocal microscopy. (c) B6-MCLs and NALP3-KO-MCLs were stained with acridine orange and subsequently treated with silica crystals as indicated. 3h after treatment cells were analyzed by flow cytometry for lysosomal acridine orange fluorescence. (d) B6-MCLs were treated with bafilomycin as indicated and stained with lysosensor green (1 µM) immediately prior to flow cytometry. (e) B6-MCLs were incubated with DQ-ovalbumin in the presence or absence of bafilomycin (250 nM) for 60 min and subjected to FACS analysis. (f) LPS-primed B6-MCLs were treated with bafilomycin or left untreated and subsequently stimulated with silica crystals or ATP. IL-1β release was measured by ELISA 6h after stimulation. Data from one representative experiment out of two are depicted.
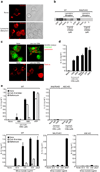
Fig. 6. Silica mediated IL-1b production is partially dependent on Cathepsin B
(a) LPS-primed B6-MCLs were treated with either cathepsin B inhibitor (CA-074-Me, 10 µM) or left untreated and subsequently stimulated with silica crystals, ATP or transfected with dAdT. IL-1β release was measured by ELISA 6h after stimulation. Data from one representative experiment out of two are depicted. (b) Bone marrow-derived macrophages of wild-type mice or NALP3-deficient mice were primed with LPS for 3h and subsequently stimulated with either silica crystals, MSU crystals, ATP or transfected with dAdT. 6h after stimulation, supernatants were analyzed for cathepsin B by Western blot. (c) B6-MCLs were incubated with silica crystals (pink) for 3 h or left untreated. Subsequently, cells were stained with fluorescent probes for activated caspase-1 (green) and activated cathepsin B (red) for one additional hour and then surface stained with fluorescent choleratoxin (blue) and analyzed by confocal microscopy.
Fig. 7. Alum activates the NALP3 inflammasome via lysosomal destablization
(b) Human PBMCs were primed with LPS (25 pg/ml) or left untreated for 3h and subsequently stimulated with alum in ascending doses. After 6h, supernatants were assessed for IL-1β production by ELISA and Western blot. ELISA data of four independent donors are depicted (upper panel) and Western blot analysis of one representative donor is shown (lower panel). (b) Bone marrow-derived macrophages of wild-type mice, NALP3-deficient mice or ASC-deficient mice were primed with LPS for 3h and subsequently stimulated with alum (500 µg/ml) and supernatants were analyzed for IL-1β by ELISA. (c) Alum (100 µg) was injected i.p. into wild-type mice (n=5) or IL1R-deficient mice (n=5), whereas PBS served as a control in wild-type mice (n=3). 16–18h after injection neutrophil counts were monitored in the peritoneal lavage by FACS. (d) B6-MCLs were incubated with 10 µg/ml DQ-ovalbumin (red) alone or together with alum (green) for 60 min, surface stained with fluorescent choleratoxin (blue) and analyzed by confocal microscopy. (e) B6-MCLs were stained with acridine orange and subsequently treated with alum (blue) as indicated. (f) B6-MCLs were incubated with alum (pink) for 3 h or left untreated. Subsequently, cells were stained with fluorescent probes for activated caspase-1 (green) and activated cathepsin B (red) for one additional hour and then surface stained with fluorescent choleratoxin (blue) and analyzed by confocal microscopy. (g) LPS-primed bone marrow-derived macrophages were treated with either cathepsin B inhibitor (CA-074-Me, 10 µM), bafilomycin (250 nM) or left untreated and subsequently stimulated with alum. IL-1β release was measured by ELISA 6h after stimulation. Data from one representative experiment out of two are depicted. (h) LPS-primed bone marrow-derived macrophages were treated with either alum or MSU in the presence of ascending doses of uricase. IL-1β release was measured by ELISA 6h after stimulation. Data were normalized to the condition without uricase.
Fig. 8. Sterile lysosomal rupture activates the NALP3 inflammasome
(a) B6-MCLs were incubated in the presence of fluorescent dextran (red) for 30 min and were left untreated or were subsequently treated using hypertonic and hypotonic solutions (see Material and Methods) to induce lysosomal rupture. (b) Bone marrow-derived macrophages of wild-type mice or NALP3-deficient mice were treated as in (a) in the presence or absence of cathepsin B inhibitor (CA-074-Me, 10 or 2 µM). In addition, ATP or dAdT were used as controls. 5h after stimulation supernatants were analyzed for actiated caspase-1. Data from one experiment out of two are depicted. (c) B6-MCLs were labeled with acridine orange (upper panel) or incubated in the presence of fluorescent dextran (red; lower panel) and incubated with Leu-Leu-OMe (1000µM). 3h after incubation cells were analyzed by confocal microscopy. (d) LPS-primed B6-MCLs were incubated with Leu-Leu-OMe (1000 or 2000 µM), silica crystals (250 µg/ml), ATP or dAdT. IL-1β release was measured by ELISA 6h after stimulation. Data from one representative experiment out of three is depicted. (e) Bone marrow-derived macrophages of wild-type mice, NALP3-deficient mice or ASC-deficient mice were primed with LPS and subsequently stimulated with Leu-Leu-OMe (500 or 1000 µM) and ATP in the presence or absence of cathepsin B inhibitor (CA-074-Me, 10 µM) or bafilomycin (250 nM) (upper panel). In addition cells were stimulated with silica crystals or dAdT in the presence or absence of cathepsin B inhibitor (CA-074-Me, 10 µM) or bafilomycin (250 nM) (lower panel).
Comment in
- NLRs and the dangers of pollution and aging.
Willingham SB, Ting JP. Willingham SB, et al. Nat Immunol. 2008 Aug;9(8):831-3. doi: 10.1038/ni0808-831. Nat Immunol. 2008. PMID: 18645588 Free PMC article.
Similar articles
- The Nalp3 inflammasome is essential for the development of silicosis.
Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. Cassel SL, et al. Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):9035-40. doi: 10.1073/pnas.0803933105. Epub 2008 Jun 24. Proc Natl Acad Sci U S A. 2008. PMID: 18577586 Free PMC article. - Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica.
Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Dostert C, et al. Science. 2008 May 2;320(5876):674-7. doi: 10.1126/science.1156995. Epub 2008 Apr 10. Science. 2008. PMID: 18403674 Free PMC article. - Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms.
Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Kuroda E, et al. Immunity. 2011 Apr 22;34(4):514-26. doi: 10.1016/j.immuni.2011.03.019. Epub 2011 Apr 14. Immunity. 2011. PMID: 21497116 - Silica-induced inflammasome activation in macrophages: role of ATP and P2X7 receptor.
Luna-Gomes T, Santana PT, Coutinho-Silva R. Luna-Gomes T, et al. Immunobiology. 2015 Sep;220(9):1101-6. doi: 10.1016/j.imbio.2015.05.004. Epub 2015 May 18. Immunobiology. 2015. PMID: 26024943 Review. - [Progress in research on role of inflammasome Nalp3 in silica dusts induced body injuries].
Zhou Y, Zhou T, Guo JL. Zhou Y, et al. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010 Oct;28(10):795-8. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010. PMID: 21126439 Review. Chinese. No abstract available.
Cited by
- Release of IL-1 β triggered by Milan summer PM10: molecular pathways involved in the cytokine release.
Bengalli R, Molteni E, Longhin E, Refsnes M, Camatini M, Gualtieri M. Bengalli R, et al. Biomed Res Int. 2013;2013:158093. doi: 10.1155/2013/158093. Epub 2013 Feb 6. Biomed Res Int. 2013. PMID: 23509682 Free PMC article. - NLRP7 and related inflammasome activating pattern recognition receptors and their function in host defense and disease.
Radian AD, de Almeida L, Dorfleutner A, Stehlik C. Radian AD, et al. Microbes Infect. 2013 Jul-Aug;15(8-9):630-9. doi: 10.1016/j.micinf.2013.04.001. Epub 2013 Apr 22. Microbes Infect. 2013. PMID: 23618810 Free PMC article. Review. - A model for homeopathic remedy effects: low dose nanoparticles, allostatic cross-adaptation, and time-dependent sensitization in a complex adaptive system.
Bell IR, Koithan M. Bell IR, et al. BMC Complement Altern Med. 2012 Oct 22;12:191. doi: 10.1186/1472-6882-12-191. BMC Complement Altern Med. 2012. PMID: 23088629 Free PMC article. - NLRP3 inflammasome activation mechanism and its role in autoimmune liver disease.
Guan Y, Gu Y, Li H, Liang B, Han C, Zhang Y, Liu Q, Wei W, Ma Y. Guan Y, et al. Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(11):1577-1586. doi: 10.3724/abbs.2022137. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36148948 Free PMC article. Review. - Genetic and epigenetic regulation of inflammasomes: Role in atherosclerosis.
Yalcinkaya M, Tall AR. Yalcinkaya M, et al. Atherosclerosis. 2024 Sep;396:118541. doi: 10.1016/j.atherosclerosis.2024.118541. Epub 2024 Jul 14. Atherosclerosis. 2024. PMID: 39111028 Review.
References
- Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. American journal of respiratory and critical care medicine. 1998;157:1666–1680. - PubMed
- Huaux F. New developments in the understanding of immunology in silicosis. Current opinion in allergy and clinical immunology. 2007;7:168–173. - PubMed
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. - PubMed
- Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Current opinion in immunology. 2007;19:615–622. - PubMed
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI083713/AI/NIAID NIH HHS/United States
- R01 AI043543/AI/NIAID NIH HHS/United States
- R01 AI-065483/AI/NIAID NIH HHS/United States
- R01 AI065483/AI/NIAID NIH HHS/United States
- R01 AI067497/AI/NIAID NIH HHS/United States
- R01 AI-067497/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases