Vaccinia virus E3 protein prevents the antiviral action of ISG15 - PubMed (original) (raw)
Vaccinia virus E3 protein prevents the antiviral action of ISG15
Susana Guerra et al. PLoS Pathog. 2008.
Abstract
The ubiquitin-like modifier ISG15 is one of the most predominant proteins induced by type I interferons (IFN). In this study, murine embryo fibroblast (MEFs) and mice lacking the gene were used to demonstrate a novel role of ISG15 as a host defense molecule against vaccinia virus (VACV) infection. In MEFs, the growth of replication competent Western Reserve (WR) VACV strain was affected by the absence of ISG15, but in addition, virus lacking E3 protein (VVDeltaE3L) that is unable to grow in ISG15+/+ cells replicated in ISG15-deficient cells. Inhibiting ISG15 with siRNA or promoting its expression in ISG15-/- cells with a lentivirus vector showed that VACV replication was controlled by ISG15. Immunoprecipitation analysis revealed that E3 binds ISG15 through its C-terminal domain. The VACV antiviral action of ISG15 and its interaction with E3 are events independent of PKR (double-stranded RNA-dependent protein kinase). In mice lacking ISG15, infection with VVDeltaE3L caused significant disease and mortality, an effect not observed in VVDeltaE3L-infected ISG15+/+ mice. Pathogenesis in ISG15-deficient mice infected with VVDeltaE3L or with an E3L deletion mutant virus lacking the C-terminal domain triggered an enhanced inflammatory response in the lungs compared with ISG15+/+-infected mice. These findings showed an anti-VACV function of ISG15, with the virus E3 protein suppressing the action of the ISG15 antiviral factor.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
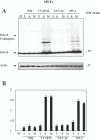
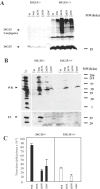
Figure 1. ISG15 protein levels are upregulated during infection of MEFs with the attenuated mutants of vaccinia virus.
A. ISG15 protein levels after WR or VVΔE3L or MVA or NYVAC infection. MEFs were mock infected (M) or infected at 5 PFU/cell with WR or MVA or NYVAC or VVΔE3L and at the indicated times p.i, cell extracts were analyzed by Western blotting. Equal amounts of proteins were fractionated by SDS-PAGE, transferred to nitrocellulose paper, and reacted with an antibody that recognizes murine ISG15 protein. On the right, the molecular weight of the proteins in kilodaltons is indicated. Actin levels showed that the same amount of protein was loaded on the gel. Uninfected cells (M) served as control. B. Densitometric quantification of ISG15 protein in arbitrary units is indicated. The graphic represents these measurements in three independent experiments.
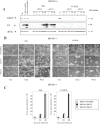
Figure 2. Effect of ISG15 on cytotoxicity and virus growth after infection of MEFs with virulent and E3L deletion VACV mutant viruses.
A. ISG15−/−, PKR−/− or wild type cells were mock-infected or infected at 0.1 PFU/cell with WR or VVΔE3L. At different times p.i, the CPE in the cells was examined by phase-contrast microscopy. B. Virus growth of WR and VVΔE3L infected (0.1 PFU/cell) ISG15+/+, or ISG15 −/−, or PKR−/− cells. At different times cells were harvested and virus yields were determined by plaque assay for WR or by immunostaining for VVΔE3L.
Figure 3. Anti VACV role of ISG15 protein expression using siRNA.
MEFs were transfected with ISG15 synthetic siRNA (siRNA1 or siRNA2), scrambled siRNA (used as a negative control) and GAPDH siRNA (used as a positive control) and 24 hours after transfection, siRNA-treated and non-treated control cells were mock- or infected with different WR or VVΔE3L viruses at 0.1 PFU/cell. A. Levels of ISG15 were measured by Western-blot using a specific murine anti-ISG15 antibody. Viral protein E3 levels were measured as a control of infection and levels of eIF2α were used as a loading control. B. CPE was visualized by phase-contrast microscopy at the indicated times p.i. C. Virus growth of WR and VVΔE3L were determined by plaque assay or by immunostaining at the indicated times p.i.
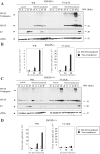
Figure 4. Effect of ISG15 overexpresion on virus growth after infection of MEFs with virulent and E3L deletion VACV mutant viruses.
A–B. ISG15−/− MEFs were transduced with high-titer viral supernatants corresponding to the pISG15-ires-GFP retroviral vector. Levels of ISG15 were measured by Western blot using a specific murine anti-ISG15 antibody. Viral protein E3 levels were measured as control of infection and levels of eIF2α were used as a loading control. Virus growth of WR and VVΔE3L were determined by plaque assay or by immunostaining. C–D. ISG15+/+ MEFs were transduced as above. Levels of ISG15 were measured by Western blot using a specific murine anti-ISG15 antibody. Viral protein E3 levels were measured as control of infection and levels of eIF2α were used as a loading control. Virus growth of WR and VVΔE3L were determined by plaque assay or by immunostaining. Control indicates the non-transduced MEFs.
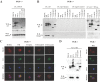
Figure 5. E3 interacts with ISG15 protein.
A. PKR+/+ MEFs were treated with mouse IFN-α (100 units/ml) during 10 hrs and then infected with 3 PFU/cell of WR, or MVA or VVΔE3L or VVE3LΔ83N or VVE3LΔ26C for 16 hours. Cell extracts were collected at 16 hpi and immunoprecipitated using anti-ISG15 serum, thoroughly washed and immunocomplexes analysed by SDS-PAGE and subjected to immunoblotting with antiserum to E3 or ISG15. B. PKR+/+ cells were treated as above and cell extracts were collected at 16 hpi and immunoprecipitated using anti-E3 (with or without RNase treatment (10 µg for 15 min at room temperature); or without antibody; or using a pre-immune serum, thoroughly washed and immunocomplexes analysed by SDS-PAGE and subjected to immunoblotting with antiserum to E3 or ISG15. C. PKR+/+ MEFs treated as in A were fixed at 16 h.p.i. and processed for immunofluorescence analysis by confocal microscopy using antibodies directed against ISG15 (red), E3 (green), and TOPRO for staining nuclei (blue). Merged images are presented in the lower panels. Cells were visualized by confocal immunofluorescence microscopy. D. Immunoprecipitation and immunoflurescence of PKR−/− MEFs was performed as in A or in C.
Figure 6. Virulence of WR or VVΔE3L after infection of ISG15+/+ and ISG15−/− mice.
A–B. Mice were inoculated i.p with purified VACV (2×107 PFU/mouse) or VVΔE3L (108 PFU/mouse). Mice were individually weighed daily, and mean percentage weight loss of each group (n = 12) was compared with the weight immediately prior to infection. C–D. Survival rate after i.p inoculation with both viruses. Dead animals were scored daily and represented as the percentage of surviving animals. P≤0.01 in all experiments. E. IL-6 measured by ELISA from serum collected at 3 hpi. Results represent the mean±SD of pooled samples from 6 mice.
Figure 7. Evaluation of extent of protection of WR or VVΔE3L pre-immunized ISG15+/+ and ISG15−/− mice after challenge with WR.
Four mice per group were first i.p, immunized with purified WR (2×107 PFU/mouse) or VVΔE3L (108 PFU/mouse), and 30 days later animals were i.n. challenged with 2×107 PFU/mouse of purified WR. A–B. Mice were individually weighed daily, and mean percentage weight loss of each group (n = 4) was compared with the weight of mice taken prior to the booster. C–D. For each mouse signs of illness, such as ruffled fur and lack of activity, were monitored with the time and are given in arbitrary units.
Figure 8. Virulence of different VACV strains after infection of ISG15+/+ and ISG15−/− mice.
A. Mice were inoculated i.n with purified WR (105 or 5×106 PFU/mouse) or VVΔE3L (5×107 PFU/mouse) or VVE3LΔ83N (5×107 PFU/mouse) or VVE3LΔ26C (5×107 PFU/mouse). Mice were individually weighed daily, and mean percentage weight loss of each group (n = 8) was compared with weight immediately prior to infection. B. Survival rate after i.n inoculation as described in A. Dead animals were scored daily and represented as the percentage of surviving animals. P≤0.01 in all experiments. C. Mice were inoculated as described above and for each individual signs of illness such as ruffled fur and lack of activity were monitored with time and are given in arbitrary units. P≤0.01 in all experiments.
Figure 9. Viral replication of different VACV strains in infected ISG15+/+ and ISG15−/− mice.
A–B. Western blot of ISG15 and of viral proteins in lung homogenates from ISG15+/+ and ISG15−/− mice. Animals were i.n, inoculated with purified WR (5×106 PFU/mouse) or VVΔE3L (5×107 PFU/mouse) or VVE3LΔ83N (5×107 PFU/mouse) or VVE3LΔ26C (5×107 PFU/mouse) and immunoblots reacted with specific antibodies to ISG15 and to viral proteins. Each sample represents pools from 2 mice per group. C. Lung homogenates were titrated by plaque assay in BSC40 (for WR) or by immunostaining in BHK-21 cells (for VVΔE3L). Each sample represents pools from 2 mice per group.
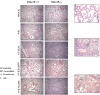
Figure 10. Histopathology of lungs from ISG15+/+ and ISG15−/− mice intranasally infected with VACV mutant viruses.
Lungs from ISG15+/+ and ISG15−/− mice i.n inoculated with, WR (5×106 PFU/mouse) or MVA (5×107 PFU/mouse) or VVΔE3L (5×107 PFU/mouse) or VVE3LΔ83N (5×107 PFU/mouse) or VVE3LΔ26C (5×107 PFU/mouse) were resected, sectioned and stained with hematoxilin and eosin. For each group of animals representative fields are shown at a magnification of 40× (left panels) and 100× (right panels). B. Bronchio, BC. Bronchiole, A. Alveolar sac, V. Vein.
Similar articles
- Host-range restriction of vaccinia virus E3L deletion mutant can be overcome in vitro, but not in vivo, by expression of the influenza virus NS1 protein.
Guerra S, Abaitua F, Martínez-Sobrido L, Esteban M, García-Sastre A, Rodríguez D. Guerra S, et al. PLoS One. 2011;6(12):e28677. doi: 10.1371/journal.pone.0028677. Epub 2011 Dec 13. PLoS One. 2011. PMID: 22174864 Free PMC article. - ISG15 regulates peritoneal macrophages functionality against viral infection.
Yángüez E, García-Culebras A, Frau A, Llompart C, Knobeloch KP, Gutierrez-Erlandsson S, García-Sastre A, Esteban M, Nieto A, Guerra S. Yángüez E, et al. PLoS Pathog. 2013;9(10):e1003632. doi: 10.1371/journal.ppat.1003632. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24137104 Free PMC article. - ISG15 is counteracted by vaccinia virus E3 protein and controls the proinflammatory response against viral infection.
Eduardo-Correia B, Martínez-Romero C, García-Sastre A, Guerra S. Eduardo-Correia B, et al. J Virol. 2014 Feb;88(4):2312-8. doi: 10.1128/JVI.03293-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257616 Free PMC article. - The antiviral activities of ISG15.
Morales DJ, Lenschow DJ. Morales DJ, et al. J Mol Biol. 2013 Dec 13;425(24):4995-5008. doi: 10.1016/j.jmb.2013.09.041. Epub 2013 Oct 3. J Mol Biol. 2013. PMID: 24095857 Free PMC article. Review. - The interferon system and vaccinia virus evasion mechanisms.
Perdiguero B, Esteban M. Perdiguero B, et al. J Interferon Cytokine Res. 2009 Sep;29(9):581-98. doi: 10.1089/jir.2009.0073. J Interferon Cytokine Res. 2009. PMID: 19708815 Review.
Cited by
- MPXV infection impairs IFN response but is partially sensitive to IFN-γ antiviral effect.
Bordi L, D'Auria A, Frasca F, Mazzotta V, Mazzetti P, Fracella M, d'Ettorre G, Antonelli G, Pistello M, Antinori A, Viscidi RP, Maggi F, Lalle E, Scagnolari C. Bordi L, et al. Med Microbiol Immunol. 2024 Nov 11;213(1):25. doi: 10.1007/s00430-024-00808-w. Med Microbiol Immunol. 2024. PMID: 39527317 - The monkeypox virus-host interplays.
Yi XM, Lei YL, Li M, Zhong L, Li S. Yi XM, et al. Cell Insight. 2024 Jul 14;3(5):100185. doi: 10.1016/j.cellin.2024.100185. eCollection 2024 Oct. Cell Insight. 2024. PMID: 39144256 Free PMC article. Review. - Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers.
Álvarez E, Falqui M, Sin L, McGrail JP, Perdiguero B, Coloma R, Marcos-Villar L, Tárrega C, Esteban M, Gómez CE, Guerra S. Álvarez E, et al. Vaccines (Basel). 2024 Feb 1;12(2):153. doi: 10.3390/vaccines12020153. Vaccines (Basel). 2024. PMID: 38400136 Free PMC article. Review. - Guanylate-Binding Protein 2 Exerts GTPase-Dependent Anti-Ectromelia Virus Effect.
Gao Z, Meng Z, He X, Chen G, Fang Y, Tian H, Zhang H, Jing Z. Gao Z, et al. Microorganisms. 2023 Sep 8;11(9):2258. doi: 10.3390/microorganisms11092258. Microorganisms. 2023. PMID: 37764102 Free PMC article. - ISG15: its roles in SARS-CoV-2 and other viral infections.
Sarkar L, Liu G, Gack MU. Sarkar L, et al. Trends Microbiol. 2023 Dec;31(12):1262-1275. doi: 10.1016/j.tim.2023.07.006. Epub 2023 Aug 10. Trends Microbiol. 2023. PMID: 37573184 Review.
References
- Liu Y, Wolff KC, Jacobs BL, Samuel CE. Vaccinia virus E3L interferon resistance protein inhibits the interferon-induced adenosine deaminase A-to-I editing activity. Virology. 2001;289:378–387. - PubMed
- Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. - PubMed
- Narasimhan J, Potter JL, Haas AL. Conjugation of the 15-kDa interferon-induced ubiquitin homolog is distinct from that of ubiquitin. J Biol Chem. 1996;271:324–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous