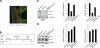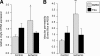Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice - PubMed (original) (raw)
Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice
Anne Granger et al. FASEB J. 2008 Oct.
Abstract
Limitation of infarct size is a major goal of therapy for acute coronary syndromes, and research has focused on achieving rapid patency of infarct-related vessels. However, new understandings of epigenetic modifications during ischemia suggest additional targeted approaches that have not been extensively explored. Here, we show that ischemia induces histone deacetylase (HDAC) activity in the heart with deacetylation of histones H3/4 in vitro and in vivo. We show, utilizing a standard murine model of ischemia-reperfusion, that chemical HDAC inhibitors significantly reduce infarct area, even when delivered 1 h after the ischemic insult. We demonstrate that HDAC inhibitors prevent ischemia-induced activation of gene programs that include hypoxia inducible factor-1alpha, cell death, and vascular permeability in vivo and in vitro, thus providing potential mechanisms to explain reduced vascular leak and myocardial injury. In vitro, siRNA knockdown experiments implicate HDAC4 as a mediator of the effects in ischemic cardiac myocytes. These results demonstrate that HDAC inhibitors alter the response to ischemic injury in the heart and reduce infarct size, suggesting novel therapeutic approaches for acute coronary syndromes.
Figures
Figure 1.
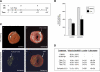
Single-dose i.p. administration of HDACIs reduces I/R-induced myocardial infarction in vivo. A) Mice were treated with either control (DMSO or Nullscript) or HDACI (TSA or Scriptaid) 1 h before ischemia (Isch) (1), 45 min after reperfusion (Rep) (2), or 12 h after reperfusion (3). After 48 h, mice were sacrificed (Sac) and quantified for infarct and area at risk (_n_=35). Rx, time of treatment protocol delivery; OR, operative maneuver. B) Cardiac extracts from the LV free wall of treated or control mice demonstrate an increase in HDAC activity with ischemia, returning to baseline levels with single-dose i.p. injections of HDACI (_n_=10). C) Quantification of the infarct size (area of infarct/area at risk). Comparison of area of infarct by TTC staining for control (1) and infarcted hearts (2). Visualization of the unaffected area perfused by fluorescent microspheres (3) and simultaneous measurements to calculate area at risk (AAR) (4). D) Control mice (DMSO or Nullscript) present consistent infarct areas, whereas mice treated either before ischemia (T1) or 45 min postreperfusion (T2) with HDACI show a marked reduction of infarct size (48.3%, _P_=0.015 to 55.6%, _P_=0.013). Treatment after 12 h (T3) is ineffective (8.3%, _P_=0.674).
Figure 2.
Inhibition of HDACs prevents deacetylation of histones H3 and H4 induced by hypoxia in isolated cardiac myocytes. A) Immunocytochemistry for MLC2v in isolated primary neonatal cardiac murine myocytes under normoxic conditions, demonstrating normal sarcomeric structure. B) Experimental protocol in vitro. Isolated cardiac myocytes were treated with either control (DMSO or Nullscript) or HDACI (TSA or Scriptaid) 1 h before hypoxia. Exposure to 5 h hypoxia was followed by reoxygenation for 90 min. C, D) Isolated cardiac myocytes were pretreated with DMSO (C) or TSA (100 nM) (D) for 1 h and exposed to 1% O2 for 5 h, followed by 90 min of reoxygenation. Cell lysates were subjected to Western blot analysis for acetylated histones H3 and H4, and relative acetylation level was estimated by densitometric quantification (_n_=3).
Figure 3.
Inhibition of HDACs promotes cell survival during hypoxia in isolated cardiac myocytes. A) Immunocytochemistry for cleaved caspase 3 in isolated cardiac myocytes. B) Quantitative determination of nuclear cleaved caspase 3 staining. *P = 0.01; 500 cells counted in 5 fields for each condition; n = 3. C, D) Live/dead cell assay performed on isolated cardiac myocytes pretreated with Nullscript or Scriptaid for 1 h and cultured under normoxic (C) or hypoxic (D) conditions for 5 h. E) Quantitative evaluation. *P = 0.04; **P = 0.01; 500 cells counted in 5 fields for each condition; n = 3.
Figure 4.
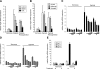
Expression analysis of in vitro ischemic cardiac myocytes reveals down-regulation of HIF1A target genes by HDACI under hypoxic conditions and coregulated gene sets. A) Neonatal cardiac myocytes were exposed to 5 h of hypoxia (1% O2), harvested, and processed for microarray analysis in biological triplicate. Lane 1: normoxia Nullscript (NN); lane 2: normoxia Scriptaid (NS); lane 3: hypoxia Nullscript (HN); lane 4: hypoxia Scriptaid (HS). Note the uniform reversal of gene expression changes by Scriptaid (lane 4) among hypoxia-induced genes (lane 3) toward their prehypoxic state (lanes 1 and 2). In addition, there were few significant changes among this group of coregulated genes with Scriptaid under normoxic conditions (lane 1 vs. 2). B) Gene profiles from the identical experiment in A made explicit, demonstrating the HIF1A-regulated genes Vegfa and Egln3. C) Venn diagram of sets of coregulated genes. The 124 genes that overlap between NN → HN and HN → HS make up the set of genes analyzed in A. D) Neonatal cardiac myocytes were pretreated with Nullscript (N) or Scriptaid (S) for 1 h and exposed to hypoxia for 5 h. Nuclear extracts were isolated and immunoblot analysis was performed with an anti-HIF1A antibody. Histone H3 was used as a nuclear loading control, and relative protein level was estimated by densitometric quantification.
Figure 5.
Inhibition of HDACs prevents hypoxia-induced increases in Vegfa expression in isolated cardiac myocytes. A, B) Cells were pretreated with DMSO/TSA (A) or Nullscript/Scriptaid (B) for 1 h and exposed to 1% O2 for 5 h. Vegfa expression levels were determined by quantitative RT-PCR. β2-microglobulin was used as an internal control. *P < 0.05 vs. control; n = 5. C, D) Cells were pretreated with increasing amounts of TSA or Scriptaid (S) for 1 h and exposed to 1% O2 for 5 h. Vegfa (C) and Egln3 (D) expression levels were determined by quantitative RT-PCR analysis to detect gene expression using specific primers. β2-microglobulin was used as an internal control. E) Cells were pretreated with Nullscript or Scriptaid for 1 h and were exposed to 1% O2 for 5 h. Egln1, Egln2, and Egln3 expression levels were determined by quantitative RT-PCR. β2-microglobulin was used as an internal control.
Figure 6.
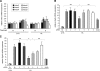
HDAC4 plays a primary role in mediating the hypoxic response of Vegfa and Egln3 in isolated cardiac myocytes. A) Cells were pretreated with Nullscript (N) or Scriptaid (S) for 1 h and exposed to 1% O2 for 5 h. Quantitative RT-PCR analysis was performed to measure gene expression levels of Hdac2, -3, -4, -5, -6, and -9. Throughout these experiments, β2-microglobulin was used as an internal control (_n_=5). B, C) Cells were transfected with scrambled siRNA (Sc, negative control) or specific siRNA directed against Hdac2, -3, -4, -5, -6, or -9. After 48 h, cells were pretreated with Nullscript or Scriptaid (SA) for 1 h and exposed to 1% O2 for 5 h. Expression levels of the HIF1A target genes Vegfa (B) and Egln3 (C) were determined by quantitative RT-PCR analysis. ns, nonsignificant. *P < 0.05; **P < 0.005; n = 4.
Figure 7.
Single-dose i.p. administration of HDACIs reduces I/R-induced Vegfa induction and vascular permeability in vivo. A) Vegfa mRNA levels from the LV free wall of treated or control mice increase with ischemia, returning to baseline levels with single-dose i.p. injections of HDACI. *P < 0.05; n = 5. B) Cardiac extracts from mice undergoing the I/R protocol treated with either vehicle (DMSO) or HDACI (TSA) were infused with Evans blue and analyzed for vascular permeability, which demonstrated a tripling of permeability with ischemia. This was subsequently halved by treatment with HDACI. **P = 0.003; n = 20.
Similar articles
- Targeted deletion of NF-kappaB p50 diminishes the cardioprotection of histone deacetylase inhibition.
Zhang LX, Zhao Y, Cheng G, Guo TL, Chin YE, Liu PY, Zhao TC. Zhang LX, et al. Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2154-63. doi: 10.1152/ajpheart.01015.2009. Epub 2010 Apr 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20382965 Free PMC article. - Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy.
Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Xie M, et al. Circulation. 2014 Mar 11;129(10):1139-51. doi: 10.1161/CIRCULATIONAHA.113.002416. Epub 2014 Jan 6. Circulation. 2014. PMID: 24396039 Free PMC article. - HDAC1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury.
Herr DJ, Baarine M, Aune SE, Li X, Ball LE, Lemasters JJ, Beeson CC, Chou JC, Menick DR. Herr DJ, et al. J Mol Cell Cardiol. 2018 Jan;114:309-319. doi: 10.1016/j.yjmcc.2017.12.004. Epub 2017 Dec 7. J Mol Cell Cardiol. 2018. PMID: 29224834 Free PMC article. - HDAC inhibition as a therapeutic strategy in myocardial ischemia/reperfusion injury.
Xie M, Tang Y, Hill JA. Xie M, et al. J Mol Cell Cardiol. 2019 Apr;129:188-192. doi: 10.1016/j.yjmcc.2019.02.013. Epub 2019 Feb 27. J Mol Cell Cardiol. 2019. PMID: 30825484 Free PMC article. Review. - Histone Deacetylase Inhibitors: A Novel Strategy for Neuroprotection and Cardioprotection Following Ischemia/Reperfusion Injury.
Pickell Z, Williams AM, Alam HB, Hsu CH. Pickell Z, et al. J Am Heart Assoc. 2020 Jun 2;9(11):e016349. doi: 10.1161/JAHA.120.016349. Epub 2020 May 22. J Am Heart Assoc. 2020. PMID: 32441201 Free PMC article. Review.
Cited by
- Activation of Autophagic Flux Blunts Cardiac Ischemia/Reperfusion Injury.
Xie M, Cho GW, Kong Y, Li DL, Altamirano F, Luo X, Morales CR, Jiang N, Schiattarella GG, May HI, Medina J, Shelton JM, Ferdous A, Gillette TG, Hill JA. Xie M, et al. Circ Res. 2021 Jul 23;129(3):435-450. doi: 10.1161/CIRCRESAHA.120.318601. Epub 2021 Jun 11. Circ Res. 2021. PMID: 34111934 Free PMC article. - Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology.
Patil RS, Maloney ME, Lucas R, Fulton DJR, Patel V, Bagi Z, Kovacs-Kasa A, Kovacs L, Su Y, Verin AD. Patil RS, et al. Biomolecules. 2024 Jan 23;14(2):140. doi: 10.3390/biom14020140. Biomolecules. 2024. PMID: 38397377 Free PMC article. Review. - Eugenol attenuates ischemia-mediated oxidative stress in cardiomyocytes via acetylation of histone at H3K27.
Randhawa PK, Rajakumar A, Futuro de Lima IB, Gupta MK. Randhawa PK, et al. Free Radic Biol Med. 2023 Jan;194:326-336. doi: 10.1016/j.freeradbiomed.2022.12.007. Epub 2022 Dec 13. Free Radic Biol Med. 2023. PMID: 36526244 Free PMC article. - gp-91 mediates histone deacetylase inhibition-induced cardioprotection.
Zhao TC, Zhang LX, Cheng G, Liu JT. Zhao TC, et al. Biochim Biophys Acta. 2010 Jul;1803(7):872-80. doi: 10.1016/j.bbamcr.2010.04.007. Epub 2010 Apr 28. Biochim Biophys Acta. 2010. PMID: 20433879 Free PMC article. - Acetyl-CoA production by specific metabolites promotes cardiac repair after myocardial infarction via histone acetylation.
Lei I, Tian S, Gao W, Liu L, Guo Y, Tang P, Chen E, Wang Z. Lei I, et al. Elife. 2021 Dec 23;10:e60311. doi: 10.7554/eLife.60311. Elife. 2021. PMID: 34939931 Free PMC article.
References
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C J, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Dallas, TX, USA: American Heart Association; Heart Disease and Stroke Statistics–2007 Update. 2006 - PubMed
- Thom T, Haase N, Rosamond W, Howard V J, Rumsfeld J, Manolio T, Zheng Z J, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff D C, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. - PubMed
- Pratt J R, Parker M D, Affleck L J, Corps C, Hostert L, Michalak E, Lodge J P. Ischemic epigenetics and the transplanted kidney. Transplant Proc. 2006;38:3344–3346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources