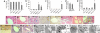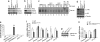IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver - PubMed (original) (raw)
IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver
Tom Luedde et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Inflammatory destruction of intrahepatic bile ducts is a common cause of vanishing bile duct syndrome and cholestasis, often progressing to biliary cirrhosis and liver failure. However, the molecular mechanisms underlying the pathogenesis of inflammatory biliary disease are poorly understood. Here, we show that the two IkappaB kinases, IKK1/IKKalpha and IKK2/IKKbeta, display distinct collaborative and specific functions that are essential to protect the liver from cytokine toxicity and bile duct disease. Combined conditional ablation of IKK1 and IKK2, but not of each kinase alone, sensitized the liver to in vivo LPS challenge, uncovering a redundant function of the two IkappaB kinases in mediating canonical NF-kappaB signaling in hepatocytes and protecting the liver from TNF-induced failure. Unexpectedly, mice with combined ablation of IKK1 and IKK2 or IKK1 and NEMO spontaneously developed severe jaundice and fatal cholangitis characterized by inflammatory destruction of small portal bile ducts. This bile duct disease was caused by the combined impairment of canonical NF-kappaB signaling together with inhibition of IKK1-specific functions affecting the bile-blood barrier. These results reveal a novel function of the two IkappaB kinases in cooperatively regulating liver immune homeostasis and bile duct integrity and suggest that IKK signaling may be implicated in human biliary diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
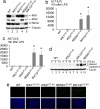
Fig. 1.
Redundant function of IKK1 and IKK2 in protecting the liver from LPS-induced toxicity. (a) Immunoblot analysis of IKK subunit expression on whole-liver protein lysates from mice with the indicated genotypes. Tubulin serves as loading control. (b and c) Liver damage 10 h after LPS administration assessed by analysis of serum ALT (b) and AST (c) levels. Error bars denote SEM (n = 3). *, P < 0.05. (d) Immunoblot analysis of caspase 3 cleavage on liver lysates prepared at the indicated time points after LPS stimulation. Tubulin serves as loading control. (e) TUNEL staining (green) on liver sections shows massive hepatocyte apoptosis in NEMOLPC-KO and IKK1/2LPC-KO mice 10 h after LPS stimulation. DAPI stains nuclei (blue). (Original magnification: ×200.)
Fig. 2.
Redundant function of IKK1 and IKK2 in TNF-induced canonical NF-κB signaling. (a) Primary hepatocytes from mice with indicated genotypes were stimulated with TNF. EMSA was performed on nuclear extracts by using an NF-κB probe. In lanes 10 and 11, antibodies for p50 or p65 were added for supershift analysis. Equal loading was assessed by EMSA, using an Oct1 probe. (b) Immunoblot analysis on total (top two panels) and nuclear protein extracts (bottom five panels) from primary hepatocytes upon TNF stimulation. Anti-PARP immunoblot was used as loading control. (c) Immunoblot analysis of primary hepatocyte extracts with antibodies against phosphorylated IκBα (_p_-IκBα) or total IκBα (top two panels). IKK complexes were immunoprecipitated with anti-NEMO antibody, and kinase activity was measured by using recombinant GST-IκBα (1–54) as a substrate (third panel from top). Equal input into the immunoprecipitation reaction was verified by tubulin immunoblot of lysates (lowest panel). (d) Immunoblot analysis of TNF-stimulated primary hepatocyte extracts with an antibody that detects p100 and processed p52. Tubulin is used as loading control. (e) Nuclear extracts from TNF-stimulated primary hepatocytes were analyzed by EMSA with an NF-κB consensus probe. In lanes 14 and 15, antibodies for p50 or p65 were added for supershift analysis. Oct1 probe was used as loading control. (f) Immunoblot analysis of protein extracts from TNF-stimulated primary hepatocytes with antibodies against phosphorylated (Ser-536) or total p65.
Fig. 3.
Mice with combined ablation of IKK1 and IKK2 in liver parenchymal cells develop spontaneous jaundice, severe cholestasis, and inflammatory destruction of small portal bile ducts. (a) Body weight analysis of 8-week-old male mice. Error bars denote SEM (n = 5). *, P < 0.05. (b–e) Analysis of serum levels of alanine aminotransferase (ALT) (b), total bilirubin (c), direct (conjugated) bilirubin (d), and alcalic phosphatase (e). Error bars denote SEM (n = 4). *, P < 0.05. (f–p) Representative liver sections stained with H&E (f–h, k–m, o, and p) or with an anti-cytokeratin 19 antibody (i, j, and n) that stains biliary epithelial cells. Portal bile ducts (indicated by yellow arrows) are present in control mice (f and i) but cannot be identified in most portal tracts in IKK1/2LPC-KO mice (g and j). Periportal hepatocytes in IKK1/2LPC-KO mice display increased positive anti-cytokeratin 19 staining as typical sign of cholestasis (j and n). (h) Tubular, “rosette”-like arrangement of hepatocytes around a dilated bile canaliculus (purple arrow) as typical histological feature of severe cholestasis (cholestatic liver cell rosettes). The blue arrow indicates a macrophage with yellow appearance (ceroid macrophage) as sign of phagocytosis of bilirubin metabolites. (k and l) Normal histological appearance of larger bile ducts in control (k) and IKK1/2LPC-KO (l) mice. (m and n) In a small number of portal tracts, remnants of small bile ducts with flattened, damaged epithelium can be identified (yellow arrows) that are often surrounded by an infiltrate containing lymphocytes (red arrow) and ceroid macrophages (blue arrow). (o and p) Representative H&E-stained liver sections from 2-day-old WT control mice (o) or IKK1/2LPC-KO mice (p) reveal normal formation of portal bile ducts at this age (yellow arrows). The portal tracts and sinusoids show extramedullary hematopoiesis, as normally seen in this stage of liver development. (Original magnification: h and n, ×400; f, g, i–m, o, and p, ×200.) (q–v) Electron microscopical analysis on livers from 8-week-old WT and IKK1/2LPC-KO mice. (q and r) Tubular arrangements of hepatocytes surrounding strongly dilated irregular lumen with disappearance of canalicular microvilli and thickened pericanalicular ectoplasma in IKK1/2LPC-KO mice (r) compared with normal canaliculus in control mice (q). Intercellular bile leakage in portal bile ducts in IKK1/2LPC-KO mice (t) showing flattened damaged epithelial cells surrounded by a thickened often doubled basement membrane compared with normal architecture in WT mice (s). In t, “BDL” indicates the bile duct lumen and “L” indicates an area of intercellular bile leakage. Biliary components are found in the lumen of cholestatic bile ducts (u) but also in the Disse space of the sinusoids (v) as signs of a disturbed junctional barrier in IKK1/2LPC-KO.
Fig. 4.
Combined inhibition of IKK1-specific functions regulating tight junction proteins in bile duct epithelium together with impairment of canonical NF-κB signaling causes bile duct disease. (a and b) Immunoblot analysis on primary hepatocyte protein extracts with indicated antibodies. (c) Primary hepatocytes were stimulated with TNF, and NF-κB DNA-binding activity was analyzed by EMSA. In lanes 10 and 11, antibodies against p50 or p65 were added for supershift analysis. This EMSA is overexposed to enhance the weak signals present in these IKK-deficient extracts. (d) Immunoblot analysis on primary hepatocyte protein extracts with an antibody recognizing full-length p100 and the cleaved form p52 (tubulin loading control). (e) Analysis of total serum bilirubin levels in mice with the indicated genotypes. Error bars denote SEM (n = 3). **, P < 0.01. (f) Relative mRNA expression of tight junction associated proteins was analyzed by quantitative RT-PCR in livers from 5-week-old mice. All values were normalized to ubiquitin expression. Cytokeratin 19 (CK-19) expression was measured to control for the presence of similar numbers of bile ducts in the samples used for analysis. Error bars denote SEM (n = 5). *, P < 0.05. (g) Claudin 23 expression was analyzed in livers from 8-week-old mice by immunoblot. (h) Relative mRNA expression of claudins 8 and 23 and of CK19 in livers from WT and IKK1/NEMOLPC-KO mice. All values were normalized to ubiquitin expression. Error bars denote SEM (n = 4). *, P < 0.05; **, P < 0.01.
Similar articles
- Zebrafish IkappaB kinase 1 negatively regulates NF-kappaB activity.
Correa RG, Matsui T, Tergaonkar V, Rodriguez-Esteban C, Izpisua-Belmonte JC, Verma IM. Correa RG, et al. Curr Biol. 2005 Jul 26;15(14):1291-5. doi: 10.1016/j.cub.2005.06.023. Curr Biol. 2005. PMID: 16051172 - Human T-cell lymphotropic virus type 1 tax induction of biologically Active NF-kappaB requires IkappaB kinase-1-mediated phosphorylation of RelA/p65.
O'Mahony AM, Montano M, Van Beneden K, Chen LF, Greene WC. O'Mahony AM, et al. J Biol Chem. 2004 Apr 30;279(18):18137-45. doi: 10.1074/jbc.M401397200. Epub 2004 Feb 12. J Biol Chem. 2004. PMID: 14963024 - NF-kappa B regulation by I kappa B kinase-2 in rheumatoid arthritis synoviocytes.
Aupperle K, Bennett B, Han Z, Boyle D, Manning A, Firestein G. Aupperle K, et al. J Immunol. 2001 Feb 15;166(4):2705-11. doi: 10.4049/jimmunol.166.4.2705. J Immunol. 2001. PMID: 11160335 - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review. - Destructive intrahepatic bile duct diseases.
Desmet VJ. Desmet VJ. Recenti Prog Med. 1990 Jun;81(6):392-8. Recenti Prog Med. 1990. PMID: 2251446 Review.
Cited by
- An NF-kappaB- and IKK-Independent Function of NEMO Prevents Hepatocarcinogenesis by Suppressing Compensatory Liver Regeneration.
Koppe C, Reisinger F, Wehr K, Vucur M, Trautwein C, Tacke F, Heikenwalder M, Luedde T. Koppe C, et al. Cancers (Basel). 2019 Jul 17;11(7):999. doi: 10.3390/cancers11070999. Cancers (Basel). 2019. PMID: 31319593 Free PMC article. - NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma.
Luedde T, Schwabe RF. Luedde T, et al. Nat Rev Gastroenterol Hepatol. 2011 Feb;8(2):108-18. doi: 10.1038/nrgastro.2010.213. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21293511 Free PMC article. Review. - Hypothesis: Targeted Ikkβ deletion upregulates MIF signaling responsiveness and MHC class II expression in mouse hepatocytes.
Koch KS, Leffert HL. Koch KS, et al. Hepat Med. 2010 Mar;2010(2):39-47. doi: 10.2147/HMER.S7208. Hepat Med. 2010. PMID: 23997575 Free PMC article. - Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases.
Pasparakis M. Pasparakis M. Nat Rev Immunol. 2009 Nov;9(11):778-88. doi: 10.1038/nri2655. Nat Rev Immunol. 2009. PMID: 19855404 Review. - Context-Dependent Role of NF-κB Signaling in Primary Liver Cancer-from Tumor Development to Therapeutic Implications.
Czauderna C, Castven D, Mahn FL, Marquardt JU. Czauderna C, et al. Cancers (Basel). 2019 Jul 25;11(8):1053. doi: 10.3390/cancers11081053. Cancers (Basel). 2019. PMID: 31349670 Free PMC article. Review.
References
- Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001;8:303–315. - PubMed
- O'Leary JG, Pratt DS. Cholestasis and cholestatic syndromes. Curr Opin Gastroenterol. 2007;23:232–236. - PubMed
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. - PubMed
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. - PubMed
- Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous