Single copies of Sec61 and TRAP associate with a nontranslating mammalian ribosome - PubMed (original) (raw)
Single copies of Sec61 and TRAP associate with a nontranslating mammalian ribosome
Jean-François Ménétret et al. Structure. 2008 Jul.
Abstract
During cotranslational protein translocation, the ribosome associates with a membrane channel, formed by the Sec61 complex, and recruits the translocon-associated protein complex (TRAP). Here we report the structure of a ribosome-channel complex from mammalian endoplasmic reticulum in which the channel has been visualized at 11 A resolution. In this complex, single copies of Sec61 and TRAP associate with a nontranslating ribosome and this stoichiometry was verified by quantitative mass spectrometry. A bilayer-like density surrounds the channel and can be attributed to lipid and detergent. The crystal structure of an archaeal homolog of the Sec61 complex was then docked into the map. In this model, two cytoplasmic loops of Sec61 may interact with RNA helices H6, H7, and H50, while the central pore is located below the ribosome tunnel exit. Hence, this copy of Sec61 is positioned to capture and translocate the nascent chain. Finally, we show that mammalian and bacterial ribosome-channel complexes have similar architectures.
Figures
Figure 1
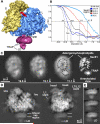
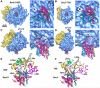
Single copies of Sec61 and TRAP are bound to a non-translating mammalian ribosome. A. The 3D map of the RCC is shown as a rendered surface with the ribosome at 8.7Å resolution and the channel region (in magenta) filtered to ∼16Å resolution. The small (S) and large (L) subunits are shown in yellow and blue. B. Resolution curves are shown for the complete RCC (light blue line), the membrane-like disk (grey) and for the Sec61 region in three maps calculated with a total of ∼24,900 (blue), 56,800 (black) and 78,800 (red) particles. C. Projections of the membrane-like disk are shown for three maps in which the ribosome was determined at resolutions of 16, 14 and 8.7Å, respectively while the Sec61 regions were imaged at 19.6, 16.1 and 11.1Å resolution (left to right, panels 1-3). Higher density is shown in white. On the far right, the denisty of the projection of the membrane-like disk has been thresholded to show that the brightest features that arise from Sec61 and TRAP in the final map (panel 4). D. Two projections are shown of the final map with the channel oriented at the bottom. The map has been filtered to ∼12Å resolution and strongly scattering material is shown in white. Small and large subunits are indicated (ssu and lsu), along with some flexible regions (head, beak of ssu and L7/L12 stalk). The density in the channel region is viewed edge-on and resembles a bilayer. The position of the TRAP lumenal domain (LD) is marked. E. Close-ups are shown of edge-on projections of the membrane-like disk in which the contrast has been adjusted to more clearly show the bilayer-like appearance.
Figure 2
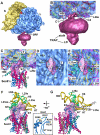
The 6/7 and 8/9 loops of Sec61 form the major connection with the ribosome. A. An oblique front view of the RCC is shown. The RCC is color coded as described in Figure 1A. The small (S) and large (L) subunits are labeled. A single major connection spans the gap between the ribosome and the channel. B. A close-up is shown of the junction between the ribosome and the membrane-like disk. The positions of connections observed in previous maps at a lower threshold (C1, C2 and C4) are indicated. Also shown are the regions of TRAP (stalk, lumenal domain, (LD)). C. A thin slab containing the interface between the ribosome and the channel is shown. Helices 50 and 7 in the large subunit interact with the loops of sec61α near the tunnel exit (T). Density for the connection is shown as a transparent surface over-layed on the modeled loops (shown as ribbons). D. A bottom view shows the insertion of the 6/7 and 8/9 loops into a pocket at the exit tunnel. The loop density is shown in magenta and the large subunit is shown in blue. The tunnel is marked with a dot and a line that points into the large subunit, towards the small subunit (yellow surface). E. This view is similar to panel 2D but the surface of the large subunit is semi-transparent, to show the atomic model of the ribosome in this region. F. The 6/7 and 8/9 loops are shown within a binding pocket which is formed by H6, H7 and H50, along with proteins L23ae, L35e and L39e. Basic residues in the Sec61 loops are shown in yellow and are labeled in the inset on the right. G. A rotated view of panel 2F is shown. A small helix of L39e is close to the 8/9 loop and L35e helps to form the back of the binding pocket.
Figure 3
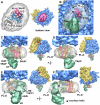
Docking the Sec61 complex into the electron density map. A. A bottom view is shown of the RCC with the membrane-embedded region rendered semi-transparent to show the docked SecY model. B. A low density Y-shaped region is present within the membrane-like disk and nearly encircles the embedded region of Sec61. The Y-like region is indicated by a dashed line. The electron density map was truncated to 17Å resolution for panels 3B and 3C. The Sec61 complex was modeled with a crystal structure of SecY and is color coded as follows. The N-terminal half of SecY/Sec61α is is colored in red, while the C-terminal half is shown in blue. The SecE/γ subunit is shown in green and the Secβ subunit is shown in tan. C. In this panel, the docked SecY in the channel region is viewed from the ribosome. D. A ribbon model of the SecY complex is shown. This view is from the ribosome and is similar to that in panels 3E and 3F. The surface helix of the SecE/Sec61γ is labeled (γ-S helix). E. A cross-section is shown of the Sec61 region from the full 3D map truncated at 11Å resolution. A thin slab encompasses the lumenal side of the channel and the SecY model fits within a low density feature (marked with dashed line). Helices of the docked model are numbered (van den Berg et al., 2004). F. A thicker slab is shown which contains the entire membrane-embedded region of Sec61.
Figure 4
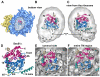
The entrance and exit vestibules of the hour-glass shaped pore in Sec61. A. The top surface of the mini-map is shown with the docked SecY model. The map has been rendered as a solid surface to show the depression leading into the pore. The 6/7 and 8/9 loops have been cut by a clipping plane to show their fit in the map. The TM of SecE/Sec61γ forms a ridge (see dashed lines). Note that the entrance and exit vestibules are similar in the complete map of the channel region. B. The same view is shown as in panel 4A, except that the surface is semi-transparent to show the helices that form the surface depression. C. The bottom surface of the mini-map is shown with the docked SecY model. The map has been rendered as a solid surface to show the depression that forms the pore exit. D. The structure in panel 4C is shown with the mini-map rendered as a semi-transparent surface. The helices which form the exit vestibule are shown.
Figure 5
A comparison of Sec61- and SecY-ribosome complexes. For panels A-D, the complete ribosome with docked Sec61 or SecY is shown on the left and a close-up is shown on the right. The color coding is yellow for the small subunit and blue for the large subunit. The N- and C-terminal halves of the Sec61α/SecY subunits are shown in blue and red ribbons respectively, while the Sec61γ/SecE and Sec61β/β-subunits are shown in green and tan. A. A bottom view is shown of the ribosome-Sec61 complex. The positions of the lateral gate (arrow) and the tunnel (*) are marked. B. A bottom view is shown of the ribosome-SecY complex with the lateral gate and tunnel marked as in panel A. C. A tilted view is shown of the ribosome-Sec61 complex in which the 6/7 and 8/9 loops are clearly visible near the tunnel exit (marked with an asterisk). D. A tilted view is shown of the ribosome-SecY complex in which the cytoplasmic loops and their insertion into the tunnel can be seen. E. The 6/7 and 8/9 loops are viewed from the exit tunnel for the Sec61-ribosome complex. The 6/7 loop is inserted between H6 and H7 while the 8/9 loop may interact with both H6 and H50. Three conserved ribosomal large subunit proteins are also shown. F. A similar view to panel E is shown of the longer 6/7 and 8/9 loops for the SecY-ribosome complex (Ménétret et al., 2007). The 6/7 loop interacts with H7 while the 8/9 loop may bind to H50 and H24.
Figure 6
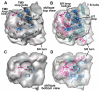
A model for TRAP in the mammalian ribosome-channel complex. A. A bottom view is shown of the membrane-like disk from the RCC (see central icon view) with the lumenal domain of TRAP removed. The disk is rendered semi-transparent and shows the docked SecY/Sec61 model within the internal low density features attributed to lipid tails. The low density region (see dashed lines) extends to the back of the disk and nearly encircles a high density region that is the correct size to contain the 7 TMs of the TRAP complex (modeled with bR, see grey ribbons). Note that Sec61 and TRAP are separated by a low density feature; hence, there is room for a row of phospholipids between them. B. This view is similar to panel A, except that TRAP is shown as a solid surface (green) and the ribosome is present. The lumenal domain is almost directly in line with the central pore of Sec61 (marked with a circle). The empty region of the disk probably contains phospholipids with digitonin at the edge (marked PL-D with curved arrows). A single arrow points from the central pore of Sec61 in the direction of the small subunit and marks the position of the proposed lateral gate. C. Modeled Sec61 and TRAP are shown in a frontal view (see icon on the right). The outline of the Sec61 molecule is shown as transparent yellow surface using the mini-map. D. A rotated view shows that empty areas in the disk on either side of Sec61 and TRAP that may contain phospholipids and detergent. E. The TRAP complex is shown from the back and has a slanted orientation. F. In a side view, there is a gap between TRAP and Sec61. The surface helix of Sec61γ may contact the membrane-embedded region of TRAP (black dot).
Figure 7
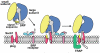
The initiation of protein translocation at the ER in higher eukaryotes. A three step model is shown for the docking of a ribosome-nascent chain-SRP complex to the ER membrane and subsequent formation of an active ribosome-channel complex (see Discussion for details).
Similar articles
- Architecture of the ribosome-channel complex derived from native membranes.
Ménétret JF, Hegde RS, Heinrich SU, Chandramouli P, Ludtke SJ, Rapoport TA, Akey CW. Ménétret JF, et al. J Mol Biol. 2005 Apr 29;348(2):445-57. doi: 10.1016/j.jmb.2005.02.053. J Mol Biol. 2005. PMID: 15811380 - Ribosome binding of a single copy of the SecY complex: implications for protein translocation.
Ménétret JF, Schaletzky J, Clemons WM Jr, Osborne AR, Skånland SS, Denison C, Gygi SP, Kirkpatrick DS, Park E, Ludtke SJ, Rapoport TA, Akey CW. Ménétret JF, et al. Mol Cell. 2007 Dec 28;28(6):1083-92. doi: 10.1016/j.molcel.2007.10.034. Mol Cell. 2007. PMID: 18158904 - Understanding the mammalian TRAP complex function(s).
Russo A. Russo A. Open Biol. 2020 May;10(5):190244. doi: 10.1098/rsob.190244. Epub 2020 May 27. Open Biol. 2020. PMID: 32453970 Free PMC article. Review. - Structure of the mammalian ribosome-Sec61 complex to 3.4 Å resolution.
Voorhees RM, Fernández IS, Scheres SH, Hegde RS. Voorhees RM, et al. Cell. 2014 Jun 19;157(7):1632-43. doi: 10.1016/j.cell.2014.05.024. Epub 2014 Jun 12. Cell. 2014. PMID: 24930395 Free PMC article. - The expanding role of the ER translocon in membrane protein folding.
Skach WR. Skach WR. J Cell Biol. 2007 Dec 31;179(7):1333-5. doi: 10.1083/jcb.200711107. J Cell Biol. 2007. PMID: 18166647 Free PMC article. Review.
Cited by
- Dengue Virus Selectively Annexes Endoplasmic Reticulum-Associated Translation Machinery as a Strategy for Co-opting Host Cell Protein Synthesis.
Reid DW, Campos RK, Child JR, Zheng T, Chan KWK, Bradrick SS, Vasudevan SG, Garcia-Blanco MA, Nicchitta CV. Reid DW, et al. J Virol. 2018 Mar 14;92(7):e01766-17. doi: 10.1128/JVI.01766-17. Print 2018 Apr 1. J Virol. 2018. PMID: 29321322 Free PMC article. - STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity.
Ishikawa H, Ma Z, Barber GN. Ishikawa H, et al. Nature. 2009 Oct 8;461(7265):788-92. doi: 10.1038/nature08476. Epub 2009 Sep 23. Nature. 2009. PMID: 19776740 Free PMC article. - Structures of membrane proteins.
Vinothkumar KR, Henderson R. Vinothkumar KR, et al. Q Rev Biophys. 2010 Feb;43(1):65-158. doi: 10.1017/S0033583510000041. Q Rev Biophys. 2010. PMID: 20667175 Free PMC article. Review. - Molecular Modeling of Signal Peptide Recognition by Eukaryotic Sec Complexes.
Bhadra P, Helms V. Bhadra P, et al. Int J Mol Sci. 2021 Oct 2;22(19):10705. doi: 10.3390/ijms221910705. Int J Mol Sci. 2021. PMID: 34639046 Free PMC article. Review. - Sss1p is required to complete protein translocon activation.
Wilkinson BM, Brownsword JK, Mousley CJ, Stirling CJ. Wilkinson BM, et al. J Biol Chem. 2010 Oct 15;285(42):32671-7. doi: 10.1074/jbc.M110.128256. Epub 2010 Aug 13. J Biol Chem. 2010. PMID: 20709746 Free PMC article.
References
- Beckmann R, Bubeck D, Grassucci R, Penczek P, Verschoor A, Blobel G, Frank J. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 1997;278:2123–2126. - PubMed
- Beckmann R, Spahn CM, Eswar N, Helmers J, Penczek PA, Sali A, Frank J, Blobel G. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 2001;107:361–372. - PubMed
- Breyton C, Haase W, Rapoport TA, Kuhlbrandt W, Collinson I. Three-dimensional structure of SecYEG, the bacterial protein-translocation complex. Nature. 2002;418:662–664. - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM045377/GM/NIGMS NIH HHS/United States
- R01 GM045377-13/GM/NIGMS NIH HHS/United States
- R01 GM045377-14/GM/NIGMS NIH HHS/United States
- R01 GM045377-15/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources