WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport - PubMed (original) (raw)
WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport
Kenneth G Campellone et al. Cell. 2008.
Abstract
The Arp2/3 complex is an actin nucleator that plays a critical role in many cellular processes. Its activities are regulated by nucleation-promoting factors (NPFs) that function primarily during plasma membrane dynamics. Here we identify a mammalian NPF called WHAMM (WASP homolog associated with actin, membranes, and microtubules) that localizes to the cis-Golgi apparatus and tubulo-vesicular membrane transport intermediates. The modular organization of WHAMM includes an N-terminal domain that mediates Golgi membrane association, a coiled-coil region that binds microtubules, and a WCA segment that stimulates Arp2/3-mediated actin polymerization. Overexpression and depletion studies indicate that WHAMM is important for maintaining Golgi structure and facilitating anterograde membrane transport. The ability of WHAMM to interact with microtubules plays a role in membrane tubulation, while its capacity to induce actin assembly promotes tubule elongation. Thus, WHAMM is an important regulator of membrane dynamics functioning at the interface of the microtubule and actin cytoskeletons.
Figures
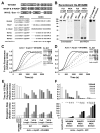
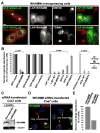
Fig.1. WHAMM is an actin nucleation-promoting factor in vitro
A. Domain organizations and sequence alignments of WHAMM and other NPFs are shown. Conserved and acidic residues are highlighted in bold. B. Purified full-length His-WHAMM and His-WCA were separated by SDS-PAGE and stained with Coomassie blue or blotted with anti-WHAMM WCA antibodies. C-D. Actin (2μM) was polymerized in the presence of 200nM full-length (FL) His-WHAMM or 20nM Arp2/3 complex +/- His-WHAMM derivatives. E-F. Relative times to half-maximal F-actin concentration and elongation rates at these times are shown in the presence of Arp2/3 and various His-NPFs.
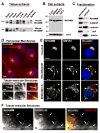
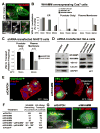
Fig.2. WHAMM associates with Golgi and ERGIC membranes and microtubules
A-B. Extracts from human and mouse organs (15μg/lane) or from Cos7, human foreskin fibroblast, NIH3T3, and HeLa cells were blotted with anti-WHAMM WCA antibodies. Anti-actin blotting was used to normalize protein content. C. Membrane and cytosolic fractions from Cos7 cells were blotted for the indicated proteins. D. Cos7 cells were stained with antibodies to the WHAMM CC domain, α-tubulin (MTs), and with DAPI. Scale bars: top, 10μm; bottom, 1μm. E. Cells were treated with a media control, nocodazole, or brefeldin A, and stained with antibodies to WHAMM, GM130, and DAPI. Arrows highlight colocalization. Scale bar: 10μm. F. Cells expressing GFP-ERGIC-53 were stained for WHAMM and GFP (pseudocolored red). Golgi positioning is indicated (G) and arrows highlight tubule colocalization. Scale bar: 10μm.
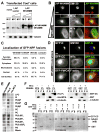
Fig.3. Distinct WHAMM domains mediate interactions with membranes and microtubules
A. Cos7 cells transfected with 35 or 150ng of LAP or LAP-WHAMM plasmids were fractionated into membranes (M) and cytosol (C) and blotted for WHAMM and α-tubulin. B. Cells transfected with 35, 100, or 250ng of LAP-WHAMM plasmid were stained for GM130 or α-tubulin (MTs). Scale bar: 10μm. Insets show a 10μm long tubulo-vesicular structure. C. Cells transfected with 100ng of NPF plasmids were stained with phalloidin and anti-α-tubulin. GFP localizations were assessed visually. The total % of cells with a given localization was determined by scoring >200 cells for each NPF in 3 experiments. D. Cells expressing intermediate levels of GFP-WHAMM truncations were stained with antibodies to GM130 or α-tubulin or with phalloidin (F-actin). Scale bar: 10μm. E. Membrane strips containing 100pmol spots of phospholipids were overlayed with His-WHAMM derivatives. Bound WHAMM was detected by anti-His blotting. F-G. His-WHAMM, His-WCA, GST, or GST-CC (200nM in panel F; 1-5μM in panel G) were centrifuged +/- microtubules (1μM in F; 2-5μM in G). Proteins in pellet (P) or supernatant (S) fractions were visualized by blotting with anti-WHAMM WCA, GST, or α-tubulin antibodies (panel F) or with Coomassie blue (panel G).
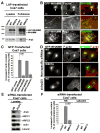
Fig.4. WHAMM-mediated actin assembly in cells requires WCA-Arp2/3 interactions
A. Cos7 cells expressing LAP-tagged variants were subjected to SDS-PAGE directly (lysates) or treated with magnetic beads to collect tagged proteins prior to SDS-PAGE (pulldowns). Proteins were visualized by blotting with antibodies to WHAMM WCA and Arp3. B. Cells expressing tagged WHAMM were stained with phalloidin (F-actin) or Arp3 antibodies. Arrows show increased F-actin content at the Golgi; arrowheads, endogenous Golgi F-actin. Scale bars: top, 10μm; middle and bottom, 1μm. C. Cells transfected with GFP-NPFs and exhibiting intermediate to high levels of GFP fluorescence were chosen randomly for examination. The phalloidin fluorescence intensity in transfected (GFP-expressing) cells was visually compared to that in nearby non-transfected cells, and the % of transfected cells with a clear increase in F-actin staining was quantified. Data are the mean +/- SEM of 2 experiments with 50 cells examined per sample. D. Cells transfected with siRNAs and LAP-WHAMM plasmids were stained with phalloidin. Scale bar: 10μm. E. Cells treated with a nonspecific (NS) control siRNA or Arp3 and ARPC4 siRNAs were blotted with antibodies to Arp3, ARPC1, and ARPC2, plus actin and α-tubulin as loading controls. F. Cells co-transfected with siRNAs and LAP plasmids were visualized as in part C, and the % with increased F-actin content was quantified. Data are the mean +/- SEM of 4 experiments with approximately 50 cells examined per sample.
Fig. 5. WHAMM overexpression or depletion disrupts Golgi structure
A. Cos7 cells expressing GFP-ERGIC-53 were transfected with mCherry or mCherry-WHAMM plasmids (left panels). Cos7 (top) and HeLa (bottom) cells overexpressing LAP-WHAMM were stained with antibodies to GM130 or TGN46. Scale bar: 10μm. B. The % of cells with a normal distribution of GFP-ERGIC-53, GM130 (_cis_-Golgi) or TGN46 (_trans_-Golgi) was quantified in Cos7 cells expressing high levels of NPFs. Data are the mean +/- SEM of 2-3 experiments with roughly 50 cells examined per sample. C. Cells treated with a nonspecific or WHAMM siRNA were blotted for WHAMM and tubulin. D. WHAMM siRNA-treated Cos7 cells were stained for WHAMM and GM130. E. The % of WHAMM-depleted or nearby non-depleted Cos7 cells with normal GM130 staining was quantified visually in a representative experiment in which >30 cells were examined per sample. Similar patterns were observed in HeLa cells (not shown). Scale bar: 10μm.
Fig. 6. WHAMM overexpression or depletion inhibits anterograde transport of VSV-G
A. Cells expressing VSV-G-GFP and shifted to 33°C for 15 min were stained with WHAMM and GFP antibodies. Scale bars: top, 10μm; bottom, 5μm. B. Cells co-expressing VSV-G-GFP and mCherry-WHAMM were shifted to 33°C for 15 or 60 min. The % with visually apparent ER, Golgi, or plasma membrane GFP fluorescence is shown for a representative experiment in which >25 cells were examined per sample. C. The % of shRNA-transfected cells (identified by their GFP fluorescence) with visually apparent Golgi or surface localization of VSV-G-mCherry was quantified and normalized to nearby non-shRNA-transfected control cells. Data are the mean +/- SEM from 2-3 experiments in which >35 cells were examined per sample. D. Cells treated with siRNAs +/- vectors encoding mCherry or siRNA-resistant mCherry-WHAMM* were transfected with VSV-G-GFP and blotted for WHAMM, GFP, tubulin, and GAPDH. E. Cells transfected with siRNAs were stained with phalloidin (F-actin). VSV-G-GFP localization to the Golgi (G) or ER is indicated. Scale bar: 10μm. F. WHAMM protein levels were determined by immunoblotting and densitometry and normalized to tubulin. The fraction of cells with punctate Golgi-like fluorescence was measured relative to parallel siGAPDH control samples. Arp2/3 data (#) are the mean of 4 experiments with approximately 60 cells examined per sample. G. Cells transfected with siRNAs were stained for WHAMM and VSV-G-GFP.
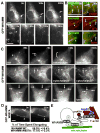
Fig. 7. WHAMM tubule dynamics require interactions with microtubules and F-actin
A. Cos7 cells expressing GFP-WHAMM were visualized by time-lapse microscopy. In all panels, arrowheads point to a fixed position and arrows indicate moving ends of dynamic tubules. Enlargements of elongating (E), contracting (C), spiraling (S), and branching (B) structures in the cell periphery away from the dense Golgi (G) are shown. Scale bars: 10μm. B. Cells expressing GFP-ERGIC53, GFP-tubulin, or mCherry-actin (all in red) and mCherry- or GFP-WHAMM (all in green) were observed. C. NIH3T3 cells expressing GFP-WHAMM were visualized before and after treatment with nocodazole, cytochalasin, or latrunculin. The bracket indicates an example of membrane dissolution. D. Cells expressing GFP-WHAMM W809A or wild type were visualized and the % of time (mean +/- SEM) that tubules spent elongating were compared (n=35 each; p<.0001). E. A model for WHAMM function during membrane tubulation is shown.
Similar articles
- WHAMM is essential for spindle formation and spindle actin polymerization in maturing mouse oocytes.
Jo YJ, Kwon J, Jin ZL, Namgoong S, Kwon T, Yoon SB, Lee DH, Kim JS, Kim NH. Jo YJ, et al. Cell Cycle. 2021 Jan;20(2):225-235. doi: 10.1080/15384101.2020.1867791. Epub 2021 Jan 5. Cell Cycle. 2021. PMID: 33397186 Free PMC article. - WHAMM Directs the Arp2/3 Complex to the ER for Autophagosome Biogenesis through an Actin Comet Tail Mechanism.
Kast DJ, Zajac AL, Holzbaur EL, Ostap EM, Dominguez R. Kast DJ, et al. Curr Biol. 2015 Jun 29;25(13):1791-7. doi: 10.1016/j.cub.2015.05.042. Epub 2015 Jun 18. Curr Biol. 2015. PMID: 26096974 Free PMC article. - RhoD regulates cytoskeletal dynamics via the actin nucleation-promoting factor WASp homologue associated with actin Golgi membranes and microtubules.
Gad AK, Nehru V, Ruusala A, Aspenström P. Gad AK, et al. Mol Biol Cell. 2012 Dec;23(24):4807-19. doi: 10.1091/mbc.E12-07-0555. Epub 2012 Oct 19. Mol Biol Cell. 2012. PMID: 23087206 Free PMC article. - WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond.
Rottner K, Hänisch J, Campellone KG. Rottner K, et al. Trends Cell Biol. 2010 Nov;20(11):650-61. doi: 10.1016/j.tcb.2010.08.014. Epub 2010 Oct 1. Trends Cell Biol. 2010. PMID: 20888769 Review. - A nucleator arms race: cellular control of actin assembly.
Campellone KG, Welch MD. Campellone KG, et al. Nat Rev Mol Cell Biol. 2010 Apr;11(4):237-51. doi: 10.1038/nrm2867. Epub 2010 Mar 18. Nat Rev Mol Cell Biol. 2010. PMID: 20237478 Free PMC article. Review.
Cited by
- Under lock and key: spatiotemporal regulation of WASP family proteins coordinates separate dynamic cellular processes.
Burianek LE, Soderling SH. Burianek LE, et al. Semin Cell Dev Biol. 2013 Apr;24(4):258-66. doi: 10.1016/j.semcdb.2012.12.005. Epub 2013 Jan 3. Semin Cell Dev Biol. 2013. PMID: 23291261 Free PMC article. Review. - Actin nucleation by WH2 domains at the autophagosome.
Coutts AS, La Thangue NB. Coutts AS, et al. Nat Commun. 2015 Jul 30;6:7888. doi: 10.1038/ncomms8888. Nat Commun. 2015. PMID: 26223951 Free PMC article. - Actin engine in immunological synapse.
Piragyte I, Jun CD. Piragyte I, et al. Immune Netw. 2012 Jun;12(3):71-83. doi: 10.4110/in.2012.12.3.71. Epub 2012 Jun 30. Immune Netw. 2012. PMID: 22916042 Free PMC article. - Membrane-deforming proteins play distinct roles in actin pedestal biogenesis by enterohemorrhagic Escherichia coli.
Campellone KG, Siripala AD, Leong JM, Welch MD. Campellone KG, et al. J Biol Chem. 2012 Jun 8;287(24):20613-24. doi: 10.1074/jbc.M112.363473. Epub 2012 Apr 27. J Biol Chem. 2012. PMID: 22544751 Free PMC article. - Actin acting at the Golgi.
Egea G, Serra-Peinado C, Salcedo-Sicilia L, Gutiérrez-Martínez E. Egea G, et al. Histochem Cell Biol. 2013 Sep;140(3):347-60. doi: 10.1007/s00418-013-1115-8. Epub 2013 Jun 27. Histochem Cell Biol. 2013. PMID: 23807268 Review.
References
- Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. - PubMed
- Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277:37771–37776. - PubMed
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–3115. - PubMed
- Cao H, Weller S, Orth JD, Chen J, Huang B, Chen JL, Stamnes M, McNiven MA. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat Cell Biol. 2005;7:483–492. - PubMed
- Carlier MF, Pantaloni D. Control of actin assembly dynamics in cell motility. J Biol Chem. 2007;282:23005–23009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases