Caspase inhibitor infusion protects an avian song control circuit from seasonal-like neurodegeneration - PubMed (original) (raw)
Caspase inhibitor infusion protects an avian song control circuit from seasonal-like neurodegeneration
Christopher K Thompson et al. J Neurosci. 2008.
Abstract
Sex steroids such as androgens and estrogens have trophic effects on the brain and can ameliorate neurodegeneration, and the withdrawal of circulating steroids induces neurodegeneration in several hormone-sensitive brain areas. Very little is known about the underlying molecular mechanisms that mediate neuronal regression caused by hormone-withdrawal, however. Here we show that reduction of programmed cell death by local infusion of caspase inhibitors rescues a telencephalic nucleus in the adult avian song control system from neurodegeneration that is induced by hormone withdrawal. This treatment also has trans-synaptic effects that provide some protection of an efferent target region. We found that unilateral infusion of caspase inhibitors in vivo in adult white-crowned sparrows rescued neurons within the hormone-sensitive song nucleus HVC (used as a proper name) from programmed cell death for as long as seven days after withdrawal of testosterone and a shift to short-day photoperiod and that the activation of caspase-3 was reduced by 59% on average in the ipsilateral HVC compared with the unmanipulated contralateral HVC. Caspase inhibitor infusion near HVC was sufficient to preserve neuron size ipsilaterally in a downstream nucleus, the robust nucleus of the arcopallium. This is the first report that sustained local application of caspase inhibitors can protect a telencephalic brain area from neurodegeneration in vivo and that a degenerating neural circuit rescued with caspase inhibitors produces sufficient trophic support to protect attributes of a downstream target that would otherwise degenerate. These results strengthen the case for the possible therapeutic use of caspase inhibitors under certain neurodegenerative conditions.
Figures
Figure 1.
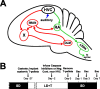
The avian song control system and timeline for experimental procedures. A, Schematic sagittal diagram of the song control system showing projections of major nuclei. The green arrows indicate the descending motor pathway. The red arrows indicate the anterior forebrain pathway. The blue arrow indicates auditory input into the song control system, which includes caudal mesopallium, caudal nidopallium, and nucleus interfacialis. B, Schematic timeline of the experimental procedures. Groups of animals that received unilateral infusion of caspase inhibitors were killed on days 1, 3, and 7. Animals that received unilateral infusion of negative control (Neg. Cont.) for caspase inhibitors were killed on day 7. DLM, Medial nucleus of the dorsolateral thalamus; nXIIts, tracheosyringeal portion of the hypoglossal motor nucleus; lMAN, lateral portion of the magnocellular nucleus of the anterior nidopallium; X, Area X; Sac, sacrifice.
Figure 2.
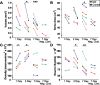
In vivo infusion of caspase inhibitors preserved ipsilateral HVC after the transition from breeding to nonbreeding conditions. Each animal within each group is represented by a distinct color across all figures. Circles represent measurements taken in ipsilateral HVC, and squares represent measurements taken in contralateral HVC. *p < 0.05, **p < 0.01, ***p < 0.005, significant differences across hemispheres (pairwise t test). A, Caspase inhibitors significantly protected ipsilateral HVC volume 3 and 7 d after transition to nonbreeding conditions. B, Caspase inhibitors significantly preserved ipsilateral HVC soma area 7 d after transition. C, Caspase inhibitors significantly reduced the increase in ipsilateral HVC neuron density 1 and 3 d after transition. D, Caspase inhibitors significantly protected ipsilateral HVC from neuron loss 3 and 7 d after transition. Infusion of a negative control for caspase inhibitors (Neg. Cont.) did not prevent regression of any attribute of HVC 7 d after the transition to nonbreeding conditions.
Figure 3.
In vivo infusion of caspase inhibitors near HVC significantly preserved soma area of ipsilateral RA neurons 7 d after the transition to nonbreeding conditions. Each animal within each group is represented by a distinct color. Circles represent measurements taken in ipsilateral RA; squares represent measurements taken in contralateral RA.*p ≤ 0.05, significant difference across hemispheres (pairwise t test).
Figure 4.
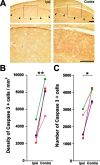
In vivo infusion of caspase inhibitors near HVC significantly reduced the activation of capsase-3 in ipsilateral HVC 3 d after the transition from breeding to nonbreeding conditions. A, Examples of immunohistochemistry for activated caspase-3 in one section taken from a 3 d bird. B, Caspase inhibitors significantly reduced the density of activated caspase-3-positive cells in ipsilateral HVC. C, Caspase inhibitors significantly reduced the number of activated caspase-3-positive cells in ipsilateral HVC. Each animal is represented by a distinct color. *p ≤ 0.05, **p ≤ 0.01, significant differences across hemispheres (pairwise t test). Scale bars, 100 μm.
Similar articles
- Neurogenesis in the adult avian song-control system.
Brenowitz EA, Larson TA. Brenowitz EA, et al. Cold Spring Harb Perspect Biol. 2015 Jun 1;7(6):a019000. doi: 10.1101/cshperspect.a019000. Cold Spring Harb Perspect Biol. 2015. PMID: 26032719 Free PMC article. Review. - Neuroprotective effects of testosterone in a naturally occurring model of neurodegeneration in the adult avian song control system.
Thompson CK, Brenowitz EA. Thompson CK, et al. J Comp Neurol. 2010 Dec 1;518(23):4760-70. doi: 10.1002/cne.22486. J Comp Neurol. 2010. PMID: 20963827 Free PMC article. - Rapamycin blocks the neuroprotective effects of sex steroids in the adult birdsong system.
Kranz TM, Lent KL, Miller KE, Chao MV, Brenowitz EA. Kranz TM, et al. Dev Neurobiol. 2019 Aug;79(8):794-804. doi: 10.1002/dneu.22719. Epub 2019 Sep 23. Dev Neurobiol. 2019. PMID: 31509642 Free PMC article. - Brain-Derived Neurotrophic Factor Has a Transsynaptic Trophic Effect on Neural Activity in an Adult Forebrain Circuit.
Miller KE, Wood WE, Brenowitz EA, Perkel DJ. Miller KE, et al. J Neurosci. 2020 Feb 5;40(6):1226-1231. doi: 10.1523/JNEUROSCI.2375-19.2019. Epub 2019 Dec 19. J Neurosci. 2020. PMID: 31857358 Free PMC article. - Seasonal-like growth and regression of the avian song control system: neural and behavioral plasticity in adult male Gambel's white-crowned sparrows.
Meitzen J, Thompson CK. Meitzen J, et al. Gen Comp Endocrinol. 2008 Jul;157(3):259-65. doi: 10.1016/j.ygcen.2008.03.014. Epub 2008 Mar 25. Gen Comp Endocrinol. 2008. PMID: 18457836 Free PMC article. Review.
Cited by
- Adult Neurogenesis Leads to the Functional Reconstruction of a Telencephalic Neural Circuit.
Cohen RE, Macedo-Lima M, Miller KE, Brenowitz EA. Cohen RE, et al. J Neurosci. 2016 Aug 24;36(34):8947-56. doi: 10.1523/JNEUROSCI.0553-16.2016. J Neurosci. 2016. PMID: 27559175 Free PMC article. - Transsynaptic trophic effects of steroid hormones in an avian model of adult brain plasticity.
Brenowitz EA. Brenowitz EA. Front Neuroendocrinol. 2015 Apr;37:119-28. doi: 10.1016/j.yfrne.2014.09.003. Epub 2014 Oct 5. Front Neuroendocrinol. 2015. PMID: 25285401 Free PMC article. Review. - Estrogen inhibits lipid peroxidation after hypoxic-ischemic brain damage in neonatal rats.
Zhu H, Han X, Ji D, Lv G, Xu M. Zhu H, et al. Neural Regen Res. 2012 Nov 5;7(31):2424-31. doi: 10.3969/j.issn.1673-5374.2012.31.003. Neural Regen Res. 2012. PMID: 25337092 Free PMC article. - Neurogenesis in the adult avian song-control system.
Brenowitz EA, Larson TA. Brenowitz EA, et al. Cold Spring Harb Perspect Biol. 2015 Jun 1;7(6):a019000. doi: 10.1101/cshperspect.a019000. Cold Spring Harb Perspect Biol. 2015. PMID: 26032719 Free PMC article. Review. - Inflammation Induced by Natural Neuronal Death and LPS Regulates Neural Progenitor Cell Proliferation in the Healthy Adult Brain.
Larson TA, Tokareva Y, Cole MM, Brenowitz EA. Larson TA, et al. eNeuro. 2020 Jul 2;7(4):ENEURO.0023-20.2020. doi: 10.1523/ENEURO.0023-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32424053 Free PMC article.
References
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. - PubMed
- Chiueh C, Lee S, Andoh T, Murphy D. Induction of antioxidative and antiapoptotic thioredoxin supports neuroprotective hypothesis of estrogen. Endocrine. 2003;21:27–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007108/GM/NIGMS NIH HHS/United States
- P30DC04661/DC/NIDCD NIH HHS/United States
- T32 DC005361/DC/NIDCD NIH HHS/United States
- P30 DC004661/DC/NIDCD NIH HHS/United States
- T32-GM07108/GM/NIGMS NIH HHS/United States
- MH53032/MH/NIMH NIH HHS/United States
- DC03829/DC/NIDCD NIH HHS/United States
- R01 MH053032/MH/NIMH NIH HHS/United States
- T32-DC05361/DC/NIDCD NIH HHS/United States
- R01 DC003829/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials