Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation - PubMed (original) (raw)
Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation
Denice M Hodgson-Zingman et al. N Engl J Med. 2008.
Abstract
Atrial fibrillation is a common arrhythmia that is hereditary in a small subgroup of patients. In a family with 11 clinically affected members, we mapped an atrial fibrillation locus to chromosome 1p36-p35 and identified a heterozygous frameshift mutation in the gene encoding atrial natriuretic peptide. Circulating chimeric atrial natriuretic peptide (ANP) was detected in high concentration in subjects with the mutation, and shortened atrial action potentials were seen in an isolated heart model, creating a possible substrate for atrial fibrillation. This report implicates perturbation of the atrial natriuretic peptide-cyclic guanosine monophosphate (cGMP) pathway in cardiac electrical instability.
2008 Massachusetts Medical Society
Figures
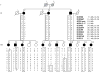
Figure 1. Pedigree of a Family with Hereditary Atrial Fibrillation
Squares indicate male subjects, and circles female subjects. Black denotes affected subjects, and white unaffected subjects; gray indicates that the status of the subject is unknown. A slash through the symbol indicates that the subject is deceased. The gene for atrial natriuretic peptide (NPPA) is located at 1p36-p35. Markers that were tested for this region of chromosome 1 are listed in order from the p-terminal end of the chromosome, with map locations according to the Web site of the National Center for Biotechnology Information (
) and given in megabases and centimorgans. A common c.454C→T polymorphism in exon 3 of wild-type NPPA is included, along with the NPPA mutation (NPPA mut). The haplotypes for these markers are shown in columns beneath family members who underwent genetic evaluation; the disease-associated haplotypes are boxed. Two subjects (III-7 and III-9) inherited portions of the disease haplotype, but not the disease gene, as a result of recombination events.
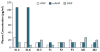
Figure 2. Radioimmunoassay Analysis Showing the Presence of Mutant ANP in Plasma from Heterozygotes for the NPPA Mutation
A radioimmunoassay with polyclonal antibodies against wild-type atrial natriuretic peptide (ANP), mutant ANP (mANP), and B-type natriuretic peptide (BNP) shows levels of circulating mANP that are 5 to 10 times higher than the levels of ANP in two family members with the NPPA mutation (in Subject III-2 during chronic atrial fibrillation and in Subject III-6 during normal sinus rhythm). In the affected subjects, plasma ANP and BNP levels are normal. Low-level cross-reactivity of the polyclonal anti-mANP antibody was observed in samples from unaffected subject (Subject III-5) and in five control subjects (three female [F] and two male [M]).
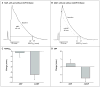
Figure 3. Electrophysiological Effects of Circulating Mutant ANP in a Rat Isolated Whole-Heart Model
In Panel A, a representative monophasic action potential (MAP) is shown at baseline and after a 40-minute perfusion with 100 nM of wild-type atrial natriuretic peptide (ANP). MAP duration at 90% repolarization (MAPD90) was not significantly changed by ANP. In Panel B, a representative MAP after a 40-minute perfusion with the mutant form of ANP (mANP) shows a shortening of MAPD90 from 47 to 41 msec. In Panel C, mANP (N = 8 hearts) and wild-type ANP (N = 10 hearts) perfusion for 40 minutes shows a significant decrease in MAPD90 for mANP as compared with the baseline value (9±2 msec, P = 0.005) but not for wild-type ANP (0.7±1.7 msec, P = 0.87). The difference in the change in MAPD90 between mANP and wild-type ANP was also significant (P = 0.008). In Panel D, mANP (N = 7 hearts) and wild-type ANP (N = 6 hearts) perfusion for 40 minutes resulted in a trend toward a reduction in the effective refractory period (ERP), as compared with the baseline value, for mANP (7.1±2.4 msec, P = 0.08) but not for wild-type ANP (P = 0.43). The difference in the change in ERP between mANP and wild-type ANP was significant (P = 0.03). The T bars denote standard errors.
Similar articles
- Electrophysiologic and molecular mechanisms of a frameshift NPPA mutation linked with familial atrial fibrillation.
Menon A, Hong L, Savio-Galimberti E, Sridhar A, Youn SW, Zhang M, Kor K, Blair M, Kupershmidt S, Darbar D. Menon A, et al. J Mol Cell Cardiol. 2019 Jul;132:24-35. doi: 10.1016/j.yjmcc.2019.05.004. Epub 2019 May 8. J Mol Cell Cardiol. 2019. PMID: 31077706 Free PMC article. - Mutation in NPPA causes atrial fibrillation by activating inflammation and cardiac fibrosis in a knock-in rat model.
Cheng C, Liu H, Tan C, Tong D, Zhao Y, Liu X, Si W, Wang L, Liang L, Li J, Wang C, Chen Q, Du Y, Wang QK, Ren X. Cheng C, et al. FASEB J. 2019 Aug;33(8):8878-8891. doi: 10.1096/fj.201802455RRR. Epub 2019 Apr 29. FASEB J. 2019. PMID: 31034774 - A familial mutation renders atrial natriuretic Peptide resistant to proteolytic degradation.
Dickey DM, Yoder AR, Potter LR. Dickey DM, et al. J Biol Chem. 2009 Jul 17;284(29):19196-202. doi: 10.1074/jbc.M109.010777. Epub 2009 May 19. J Biol Chem. 2009. PMID: 19458086 Free PMC article. - The role of atrial natriuretic peptide in modulating cardiac electrophysiology.
Perrin MJ, Gollob MH. Perrin MJ, et al. Heart Rhythm. 2012 Apr;9(4):610-5. doi: 10.1016/j.hrthm.2011.11.019. Epub 2011 Nov 12. Heart Rhythm. 2012. PMID: 22083030 Review. - Depletion of atrial natriuretic peptide during longstanding atrial fibrillation.
van den Berg MP, van Gelder IC, van Veldhuisen DJ. van den Berg MP, et al. Europace. 2004 Sep;6(5):433-7. doi: 10.1016/j.eupc.2004.04.006. Europace. 2004. PMID: 15294268 Review.
Cited by
- Cardiac corin and atrial natriuretic peptide regulate liver glycogen metabolism and glucose homeostasis.
Li W, Zhang X, Zhou Z, Guo W, Wang M, Zhou T, Liu M, Wu Q, Dong N. Li W, et al. Cardiovasc Diabetol. 2024 Oct 28;23(1):383. doi: 10.1186/s12933-024-02475-w. Cardiovasc Diabetol. 2024. PMID: 39468553 Free PMC article. - Personalized medicine and atrial fibrillation: will it ever happen?
Lubitz SA, Ellinor PT. Lubitz SA, et al. BMC Med. 2012 Dec 4;10:155. doi: 10.1186/1741-7015-10-155. BMC Med. 2012. PMID: 23210687 Free PMC article. Review. - Can we predict the occurrence of atrial fibrillation?
Schnabel RB. Schnabel RB. Clin Cardiol. 2012 Jan;35 Suppl 1(Suppl 1):5-9. doi: 10.1002/clc.20963. Clin Cardiol. 2012. PMID: 22246951 Free PMC article. Review. - Genetics of atrial fibrillation: from families to genomes.
Christophersen IE, Ellinor PT. Christophersen IE, et al. J Hum Genet. 2016 Jan;61(1):61-70. doi: 10.1038/jhg.2015.44. Epub 2015 May 21. J Hum Genet. 2016. PMID: 25994868 Review. - EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication.
Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S. Goette A, et al. Heart Rhythm. 2017 Jan;14(1):e3-e40. doi: 10.1016/j.hrthm.2016.05.028. Epub 2016 Jun 10. Heart Rhythm. 2017. PMID: 27320515 Free PMC article. Review. No abstract available.
References
- Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. - PubMed
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. - PubMed
- Fox CS, Parise H, D’Agostino RB, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–5. - PubMed
- Gudbjartsson DF, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–7. - PubMed
- Brugada R, Tapscott T, Czernuszewicz GZ, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL075495-01A2/HL/NHLBI NIH HHS/United States
- K23 HL075266/HL/NHLBI NIH HHS/United States
- P01 HL76611/HL/NHLBI NIH HHS/United States
- R01 HL036634/HL/NHLBI NIH HHS/United States
- P01 HL076611/HL/NHLBI NIH HHS/United States
- R01 HL075495-02/HL/NHLBI NIH HHS/United States
- K23 HL075266-04/HL/NHLBI NIH HHS/United States
- R01 HL36634/HL/NHLBI NIH HHS/United States
- R01 HL075495/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials