Cortactin branches out: roles in regulating protrusive actin dynamics - PubMed (original) (raw)
Review
Cortactin branches out: roles in regulating protrusive actin dynamics
Amanda Gatesman Ammer et al. Cell Motil Cytoskeleton. 2008 Sep.
Abstract
Since its discovery in the early 1990's, cortactin has emerged as a key signaling protein in many cellular processes, including cell adhesion, migration, endocytosis, and tumor invasion. While the list of cellular functions influenced by cortactin grows, the ability of cortactin to interact with and alter the cortical actin network is central to its role in regulating these processes. Recently, several advances have been made in our understanding of the interaction between actin and cortactin, providing insight into how these two proteins work together to provide a framework for normal and altered cellular function. This review examines how regulation of cortactin through post-translational modifications and interactions with multiple binding partners elicits changes in cortical actin cytoskeletal organization, impacting the regulation and formation of actin-rich motility structures.
Figures
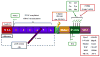
Figure 1
Domain structure of cortactin and associated binding proteins. Specific cortactin domains are described in the text. Proteins outlined in red represent binding partners with known interaction sites. Proteins outlined in blue represent known binding partners with undetermined binding sites. Proteins outlined in green represent kinases known to phosphorylate cortactin at the indicated sites.
Figure 2
Cortactin-mediated activation of Arp2/3 actin nucleation and phosphorylation-independent interactions with N-WASp and WIP. A. Cortactin functions as an NPF for Arp2/3 complex. Cortactin binds the Arp2/3 through its NTA domain and F-actin through the fourth tandem repeat, resulting in direct activation Arp2/3 actin nucleation activity. B. Cortactin activates N-WASp. Cortactin enhances N-WASp mediated Arp2/3 activation by disrupting N-WASp autoinhibition through binding of the cortactin SH3 domain to the PRR region of N-WASp. The CA domain of N-WASp binds to and activates Arp2/3, while the WH2 domain binds ATP-loaded G-actin. In this scenario, N-WASp appears to be the dominant activator of Arp2/3 complex. C. WIP enhances cortactin-mediated Arp2/3 activation. The cortactin SH3 domain interacts with the WIP cortactin binding domain (CBD), presumably bringing tandem WH2 domains with bound G-actin in close proximity with Arp2/3 complex activated by the cortactin NTA, serving to enhance cortactin-mediated Arp2/3 activation.
Figure 2
Cortactin-mediated activation of Arp2/3 actin nucleation and phosphorylation-independent interactions with N-WASp and WIP. A. Cortactin functions as an NPF for Arp2/3 complex. Cortactin binds the Arp2/3 through its NTA domain and F-actin through the fourth tandem repeat, resulting in direct activation Arp2/3 actin nucleation activity. B. Cortactin activates N-WASp. Cortactin enhances N-WASp mediated Arp2/3 activation by disrupting N-WASp autoinhibition through binding of the cortactin SH3 domain to the PRR region of N-WASp. The CA domain of N-WASp binds to and activates Arp2/3, while the WH2 domain binds ATP-loaded G-actin. In this scenario, N-WASp appears to be the dominant activator of Arp2/3 complex. C. WIP enhances cortactin-mediated Arp2/3 activation. The cortactin SH3 domain interacts with the WIP cortactin binding domain (CBD), presumably bringing tandem WH2 domains with bound G-actin in close proximity with Arp2/3 complex activated by the cortactin NTA, serving to enhance cortactin-mediated Arp2/3 activation.
Figure 3
Increased complexity and role of tyrosine phosphorylation in cortactin-mediated Arp2/3 complex activation. A. Activation of Arp2/3 complex by a cortactin/WIP/N-WASp complex. Binding of the cortactin SH3 domain to the CBD of WIP, coupled with the interaction of the WIP WASp binding domain (WBD) to the WH1 domain of N-WASp creates a trimeric complex capable of activating Arp2/3 actin nucleation activity. B. Tyrosine phosphorylation of cortactin influences Arp2/3 activation. The adaptor NCK binds cortactin through an SH2-phosphotyrosine mediated interaction. An SH3 domain of Nck1 in turn interacts with proline-rich regions on WIP, thereby positioning WIP to enhance Arp2/3 activation in an analogous manner to the positioning of WIP in Fig. 2C. C. N-WASP induced Arp2/3 activation mediated by an Nck1/cortactin complex. Nck1 binding to tyrosine phosphorylated cortactin as in B can also interact with and activate N-WASp through binding of one or more Nck1 SH3 domains binding to the proline rich region (PRR) of N-WASp, resulting in Arp2/3 activation. D. Multiprotein complexes of tyrosine phosphorylated cortactin, Nck1, N-WASp and WIP incorporating aspects of Fig 2 and 3 are conceivable, with the possible binding of WIP to the cortactin SH3 domain and/or N-WASp in addition to the Nck1/N-WASp complex described in C. In instances where N-WASp is present, cortactin appears to function as an adaptor rather than an NPF. Abbreviations: N-WASp-WH1: WASP-homology 1; GBD: GTPase-binding domain; PRR: proline-rich region; W: WASP-homology 2 domain (WH2); CA: Arp2/3 binding connector and acidic domain. WIP – CBD: cortactin binding domain; NBD: Nck binding domain; WBD: N-WASp binding domain. Nck1 – SH3: Src-homology 3 domain; SH2: Src-homology 2 domain. Cortactin – NTA: N-terminal acidic domain; Helix: alpha-helical region; P-rich: proline-rich region. For simplicity, actin filaments are shown where the Arp2/3 complex is located to demonstrate nucleation activity.
Figure 3
Increased complexity and role of tyrosine phosphorylation in cortactin-mediated Arp2/3 complex activation. A. Activation of Arp2/3 complex by a cortactin/WIP/N-WASp complex. Binding of the cortactin SH3 domain to the CBD of WIP, coupled with the interaction of the WIP WASp binding domain (WBD) to the WH1 domain of N-WASp creates a trimeric complex capable of activating Arp2/3 actin nucleation activity. B. Tyrosine phosphorylation of cortactin influences Arp2/3 activation. The adaptor NCK binds cortactin through an SH2-phosphotyrosine mediated interaction. An SH3 domain of Nck1 in turn interacts with proline-rich regions on WIP, thereby positioning WIP to enhance Arp2/3 activation in an analogous manner to the positioning of WIP in Fig. 2C. C. N-WASP induced Arp2/3 activation mediated by an Nck1/cortactin complex. Nck1 binding to tyrosine phosphorylated cortactin as in B can also interact with and activate N-WASp through binding of one or more Nck1 SH3 domains binding to the proline rich region (PRR) of N-WASp, resulting in Arp2/3 activation. D. Multiprotein complexes of tyrosine phosphorylated cortactin, Nck1, N-WASp and WIP incorporating aspects of Fig 2 and 3 are conceivable, with the possible binding of WIP to the cortactin SH3 domain and/or N-WASp in addition to the Nck1/N-WASp complex described in C. In instances where N-WASp is present, cortactin appears to function as an adaptor rather than an NPF. Abbreviations: N-WASp-WH1: WASP-homology 1; GBD: GTPase-binding domain; PRR: proline-rich region; W: WASP-homology 2 domain (WH2); CA: Arp2/3 binding connector and acidic domain. WIP – CBD: cortactin binding domain; NBD: Nck binding domain; WBD: N-WASp binding domain. Nck1 – SH3: Src-homology 3 domain; SH2: Src-homology 2 domain. Cortactin – NTA: N-terminal acidic domain; Helix: alpha-helical region; P-rich: proline-rich region. For simplicity, actin filaments are shown where the Arp2/3 complex is located to demonstrate nucleation activity.
Figure 3
Increased complexity and role of tyrosine phosphorylation in cortactin-mediated Arp2/3 complex activation. A. Activation of Arp2/3 complex by a cortactin/WIP/N-WASp complex. Binding of the cortactin SH3 domain to the CBD of WIP, coupled with the interaction of the WIP WASp binding domain (WBD) to the WH1 domain of N-WASp creates a trimeric complex capable of activating Arp2/3 actin nucleation activity. B. Tyrosine phosphorylation of cortactin influences Arp2/3 activation. The adaptor NCK binds cortactin through an SH2-phosphotyrosine mediated interaction. An SH3 domain of Nck1 in turn interacts with proline-rich regions on WIP, thereby positioning WIP to enhance Arp2/3 activation in an analogous manner to the positioning of WIP in Fig. 2C. C. N-WASP induced Arp2/3 activation mediated by an Nck1/cortactin complex. Nck1 binding to tyrosine phosphorylated cortactin as in B can also interact with and activate N-WASp through binding of one or more Nck1 SH3 domains binding to the proline rich region (PRR) of N-WASp, resulting in Arp2/3 activation. D. Multiprotein complexes of tyrosine phosphorylated cortactin, Nck1, N-WASp and WIP incorporating aspects of Fig 2 and 3 are conceivable, with the possible binding of WIP to the cortactin SH3 domain and/or N-WASp in addition to the Nck1/N-WASp complex described in C. In instances where N-WASp is present, cortactin appears to function as an adaptor rather than an NPF. Abbreviations: N-WASp-WH1: WASP-homology 1; GBD: GTPase-binding domain; PRR: proline-rich region; W: WASP-homology 2 domain (WH2); CA: Arp2/3 binding connector and acidic domain. WIP – CBD: cortactin binding domain; NBD: Nck binding domain; WBD: N-WASp binding domain. Nck1 – SH3: Src-homology 3 domain; SH2: Src-homology 2 domain. Cortactin – NTA: N-terminal acidic domain; Helix: alpha-helical region; P-rich: proline-rich region. For simplicity, actin filaments are shown where the Arp2/3 complex is located to demonstrate nucleation activity.
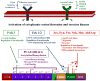
Figure 4
Schematic illustration of membrane- and cytoplasmic-based signaling molecules and their post-translational modifications on cortactin function. Kinase-based signaling cascades initiated by adhesion or growth factor receptors are mediated by select serine/threonine and tyrosine kinases that serve to modify cortactin at the indicated amino acids. Cortactin function is also regulated by a cycle of acetylation/deacetylation. Functional consequences of each specific modification are indicated.
Similar articles
- Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence.
Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Kelley LC, et al. PLoS One. 2010 Nov 4;5(11):e13847. doi: 10.1371/journal.pone.0013847. PLoS One. 2010. PMID: 21079800 Free PMC article. - Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control.
Lua BL, Low BC. Lua BL, et al. FEBS Lett. 2005 Jan 31;579(3):577-85. doi: 10.1016/j.febslet.2004.12.055. FEBS Lett. 2005. PMID: 15670811 Review. - A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin.
Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. Tian L, et al. FASEB J. 2006 Dec;20(14):2588-90. doi: 10.1096/fj.06-6152fje. Epub 2006 Oct 25. FASEB J. 2006. PMID: 17065230 - Supervillin reorganizes the actin cytoskeleton and increases invadopodial efficiency.
Crowley JL, Smith TC, Fang Z, Takizawa N, Luna EJ. Crowley JL, et al. Mol Biol Cell. 2009 Feb;20(3):948-62. doi: 10.1091/mbc.e08-08-0867. Epub 2008 Dec 24. Mol Biol Cell. 2009. PMID: 19109420 Free PMC article. - Cortactin: a multifunctional regulator of cellular invasiveness.
Kirkbride KC, Sung BH, Sinha S, Weaver AM. Kirkbride KC, et al. Cell Adh Migr. 2011 Mar-Apr;5(2):187-98. doi: 10.4161/cam.5.2.14773. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21258212 Free PMC article. Review.
Cited by
- Cortactin as a target for FAK in the regulation of focal adhesion dynamics.
Tomar A, Lawson C, Ghassemian M, Schlaepfer DD. Tomar A, et al. PLoS One. 2012;7(8):e44041. doi: 10.1371/journal.pone.0044041. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952866 Free PMC article. - Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion.
Oikawa T, Oyama M, Kozuka-Hata H, Uehara S, Udagawa N, Saya H, Matsuo K. Oikawa T, et al. J Cell Biol. 2012 May 14;197(4):553-68. doi: 10.1083/jcb.201111116. J Cell Biol. 2012. PMID: 22584907 Free PMC article. - 5'-Inositol phosphatase SHIP2 recruits Mena to stabilize invadopodia for cancer cell invasion.
Rajadurai CV, Havrylov S, Coelho PP, Ratcliffe CD, Zaoui K, Huang BH, Monast A, Chughtai N, Sangwan V, Gertler FB, Siegel PM, Park M. Rajadurai CV, et al. J Cell Biol. 2016 Sep 12;214(6):719-34. doi: 10.1083/jcb.201501003. Epub 2016 Sep 5. J Cell Biol. 2016. PMID: 27597754 Free PMC article. - Detoxification, Hydrogen Sulphide Metabolism and Wound Healing Are the Main Functions That Differentiate Caecum Protein Expression from Ileum of Week-Old Chicken.
Volf J, Rajova J, Babak V, Seidlerova Z, Rychlik I. Volf J, et al. Animals (Basel). 2021 Nov 4;11(11):3155. doi: 10.3390/ani11113155. Animals (Basel). 2021. PMID: 34827887 Free PMC article. - Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo.
Schnoor M, Lai FP, Zarbock A, Kläver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Schnoor M, et al. J Exp Med. 2011 Aug 1;208(8):1721-35. doi: 10.1084/jem.20101920. Epub 2011 Jul 25. J Exp Med. 2011. PMID: 21788407 Free PMC article.
References
- Anton IM, Jones GE. WIP: a multifunctional protein involved in actin cytoskeleton regulation. Eur J Cell Biol. 2006;85(3–4):295–304. - PubMed
- Anton IM, Saville SP, Byrne MJ, Curcio C, Ramesh N, Hartwig JH, Geha RS. WIP participates in actin reorganization and ruffle formation induced by PDGF. J Cell Sci. 2003;116(Pt 12):2443–51. - PubMed
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66(6):3034–43. - PubMed
- Aspenstrom P. The mammalian verprolin homologue WIRE participates in receptor-mediated endocytosis and regulation of the actin filament system by distinct mechanisms. Exp Cell Res. 2004;298(2):485–98. - PubMed
- Ayala I, Baldassarre M, Caldieri G, Buccione R. Invadopodia: a guided tour. Eur J Cell Biol. 2006;85(3–4):159–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR16440/RR/NCRR NIH HHS/United States
- P20 RR016440/RR/NCRR NIH HHS/United States
- P20 RR016440-076908/RR/NCRR NIH HHS/United States
- R01 DE014578-06/DE/NIDCR NIH HHS/United States
- R01 DE014578/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources