Memantine leads to behavioral improvement and amyloid reduction in Alzheimer's-disease-model transgenic mice shown as by micromagnetic resonance imaging - PubMed (original) (raw)
Comparative Study
. 2008 Sep;86(12):2784-91.
doi: 10.1002/jnr.21713.
Affiliations
- PMID: 18615702
- PMCID: PMC2723808
- DOI: 10.1002/jnr.21713
Comparative Study
Memantine leads to behavioral improvement and amyloid reduction in Alzheimer's-disease-model transgenic mice shown as by micromagnetic resonance imaging
Henrieta Scholtzova et al. J Neurosci Res. 2008 Sep.
Abstract
Memantine, an N-methyl-D-aspartate (NMDA) receptor antagonist, has been shown to improve learning and memory in several preclinical models of Alzheimer's disease (AD). Memantine has also been shown to reduce the levels of amyloid beta (A beta) peptides in human neuroblastoma cells as well as to inhibit A beta oligomer-induced synaptic loss. In this study, we assessed whether NMDA receptor inhibition by memantine in transgenic mice expressing human amyloid-beta precursor protein (APP) and presenilin 1 (PS1) is associated with cognitive benefit and amyloid burden reduction by using object recognition, micromagnetic resonance imaging (micro MRI), and histology. APP/PS1 Tg mice were treated either with memantine or with vehicle for a period of 4 months starting at 3 months of age. After treatment, the mice were subjected to an object recognition test and analyzed by ex vivo micro MRI, and histological examination of amyloid burden. micro MRI was performed following injection with gadolinium-DTPA-A beta(1-40). We found that memantine-treated Tg mice performed the same as wild-type control mice, whereas the performance of vehicle-treated Tg mice was significantly impaired (P = 0.0081, one-way ANOVA). Compared with vehicle-treated animals, memantine-treated Tg mice had a reduced plaque burden, as determined both histologically and by micro MRI. This reduction in amyloid burden correlates with an improvement in cognitive performance. Thus, our findings provide further evidence of the potential role of NMDA receptor antagonists in ameliorating AD-related pathology. In addition, our study shows, for the first time, the utility of micro MRI in conjunction with gadolinium-labeled A beta labeling agents to monitor the therapeutic response to amyloid-reducing agents.
(c) 2008 Wiley-Liss, Inc.
Figures
Fig. 1
Memantine significantly improved short-term memory in APP/PS1 mice. At 7 months of age (posttreatment), memantine-treated APP/PS1 mice performed as well as wild-type mice and significantly better than vehicle-treated APP/PS1 mice in a novel-object recognition test (★★ P = 0.008 compared with vehicle-APP/PS1 mice; one-way ANOVA). The exploration difference (%) was calculated by subtracting the percentage time spent by mice exploring the novel object from the percentage time spent exploring the familiar object (Bars indicate mean ± standard error of the mean).
Fig. 2
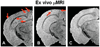
Memantine decreased amyloid plaque burden in APP/PS1 mice. Micro-MRI scans of vehicle-treated APP/PS1 mice (A), memantine-treated APP/PS1 mice (B), and age-matched wild-type mice (C) at 7 months revealed fewer amyloid plaques (red arrows) in memantine-treated APP/PS1 mice compared with vehicle-treated APP/PS1 mice. Aβ plaques were detected with ex vivo µMRI after intracarotid injection of Gd-DTPA-Aβ1–40 peptide with manitol.
Fig. 3
Average cortical T2★ values in treated vs. control APP/PS1 Tg mice. Quantification of cortical absolute T2★ values also demonstrated a memantine amyloid burden-lowering effect (★ P = 0.04; _t_-test) (Bars indicate mean ± standard error of the mean).
Fig. 4
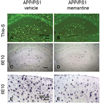
Memantine decreased hippocampal and cortical plaque burden in APP/PS1 mice. Histological analysis of APP/PS1–21 Tg mice showed the difference in Aβ burden. Thioflavin-S staining revealed more amyloid plaques in hippocampal sections of vehicle-treated APP/PS1 mice (A) compared with memantine-treated APP/PS1 mice (B). Similarly, Aβ immunostaining showed greater Aβ accumulation in hippocampal sections of vehicle-treated APP/PS1 mice (C) compared with sections from memantine-treated APP/PS1 mice (D). Cortical Aβ immunoreactivity also revealed differences between vehicle-treated (E) and memantine-treated (F) APP/PS1 mice. Scale bars = 200 µm.
Fig. 5
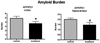
Memantine reduced amyloid burden in APP/PS1 mice. Significant reduction in the area covered by Aβ (Aβ load) was observed in APP/PS1-Tg mice treated with memantine compared with age-matched Tg contol mice treated with vehicle. There was a 25% reduction in cortical amyloid burden (★ P = 0.047, two-tailed t-test) and a 28% reduction in hippocampal amyloid burden (# P = 0.021) as quantified by using an unbiased random sampling scheme and semiautomated image analysis system (Bars indicate mean ± standard error of the mean).
Fig. 6
Memantine reduced cortical and hippocampal CD45 microglia in APP/PS1 mice. CD45 immunostaining followed by stereological analysis revealed fewer activated microglia in memantine-treated Tg animals compared with vehicle-treated animals, but the difference was not statistically significant (Bars indicate mean ± standard error of the mean).
Fig. 7
Memantine reduced cortical and hippocampal GFAP in APP/PS1 mice. GFAP immunostaining followed by stereological analysis revealed fewer activated astrocytes in memantine-treated Tg animals compared with vehicle-treated animals, but the difference was not statistically significant (Bars indicate mean ± standard error of the mean).
Similar articles
- False recognition in a mouse model of Alzheimer's disease: rescue with sensory restriction and memantine.
Romberg C, McTighe SM, Heath CJ, Whitcomb DJ, Cho K, Bussey TJ, Saksida LM. Romberg C, et al. Brain. 2012 Jul;135(Pt 7):2103-14. doi: 10.1093/brain/aws074. Epub 2012 Mar 30. Brain. 2012. PMID: 22466291 Free PMC article. - Characterization of cognitive deficits in a transgenic mouse model of Alzheimer's disease and effects of donepezil and memantine.
Nagakura A, Shitaka Y, Yarimizu J, Matsuoka N. Nagakura A, et al. Eur J Pharmacol. 2013 Mar 5;703(1-3):53-61. doi: 10.1016/j.ejphar.2012.12.023. Epub 2012 Dec 28. Eur J Pharmacol. 2013. PMID: 23276665 - Effects of the drug combination memantine and melatonin on impaired memory and brain neuronal deficits in an amyloid-predominant mouse model of Alzheimer's disease.
Jürgenson M, Zharkovskaja T, Noortoots A, Morozova M, Beniashvili A, Zapolski M, Zharkovsky A. Jürgenson M, et al. J Pharm Pharmacol. 2019 Nov;71(11):1695-1705. doi: 10.1111/jphp.13165. Epub 2019 Sep 18. J Pharm Pharmacol. 2019. PMID: 31531878 - The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease.
Rogawski MA, Wenk GL. Rogawski MA, et al. CNS Drug Rev. 2003 Fall;9(3):275-308. doi: 10.1111/j.1527-3458.2003.tb00254.x. CNS Drug Rev. 2003. PMID: 14530799 Free PMC article. Review. - Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine--searching for the connections.
Danysz W, Parsons CG. Danysz W, et al. Br J Pharmacol. 2012 Sep;167(2):324-52. doi: 10.1111/j.1476-5381.2012.02057.x. Br J Pharmacol. 2012. PMID: 22646481 Free PMC article. Review.
Cited by
- Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer's disease transgenic mice using magnetic resonance microimaging.
Wadghiri YZ, Li J, Wang J, Hoang DM, Sun Y, Xu H, Tsui W, Li Y, Boutajangout A, Wang A, de Leon M, Wisniewski T. Wadghiri YZ, et al. PLoS One. 2013;8(2):e57097. doi: 10.1371/journal.pone.0057097. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23468919 Free PMC article. - Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer's disease.
Yue Q, Hoi MPM. Yue Q, et al. Neural Regen Res. 2023 Sep;18(9):1890-1902. doi: 10.4103/1673-5374.367832. Neural Regen Res. 2023. PMID: 36926705 Free PMC article. Review. - AD vaccines: conclusions and future directions.
Wisniewski T. Wisniewski T. CNS Neurol Disord Drug Targets. 2009 Apr;8(2):160-6. doi: 10.2174/187152709787847289. CNS Neurol Disord Drug Targets. 2009. PMID: 19355935 Free PMC article. Review. No abstract available. - Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice.
Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK. Alley GM, et al. J Neurosci Res. 2010 Jan;88(1):143-54. doi: 10.1002/jnr.22172. J Neurosci Res. 2010. PMID: 19642202 Free PMC article. - Sex Differences in Psychiatric Disease: A Focus on the Glutamate System.
Wickens MM, Bangasser DA, Briand LA. Wickens MM, et al. Front Mol Neurosci. 2018 Jun 5;11:197. doi: 10.3389/fnmol.2018.00197. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29922129 Free PMC article. Review.
References
- Asuni A, Boutajangout A, Scholtzova H, Knudsen E, Li Y, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Aβ derivative vaccination in alum adjuvant prevents amyloid deposition and does not cause brain microhemorrhages in Alzheimer’s model mice. Eur J Neurosci. 2006;24:2530–2542. - PMC - PubMed
- Banerjee PK, Lahiri DK, Tanila H, Miguel-Hidalgo JJ, Iqbal K. Preclinical basis for the efficacy of memantine in Alzheimer’s disease. Biol Psychiatry. 2005;57:173S–174S.
- Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci. 1996;8:565–571. - PubMed
- Blennow K, De Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. - PubMed
- Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer’s disease. J Alzheimers Dis. 2006;10:81–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG020245/AG/NIA NIH HHS/United States
- R01 AG015408-09/AG/NIA NIH HHS/United States
- R01 AG032611/AG/NIA NIH HHS/United States
- R01 AG020197/AG/NIA NIH HHS/United States
- AG15408/AG/NIA NIH HHS/United States
- R01 AG020245-07/AG/NIA NIH HHS/United States
- AG20245/AG/NIA NIH HHS/United States
- R01 AG015408/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous