Demonstration of a multistep mechanism for assembly of the SRP x SRP receptor complex: implications for the catalytic role of SRP RNA - PubMed (original) (raw)
Demonstration of a multistep mechanism for assembly of the SRP x SRP receptor complex: implications for the catalytic role of SRP RNA
Xin Zhang et al. J Mol Biol. 2008.
Abstract
Two GTPases in the signal recognition particle (SRP) and its receptor (SR) control the delivery of newly synthesized proteins to the endoplasmic reticulum or plasma membrane. During the protein targeting reaction, the 4.5S SRP RNA accelerates the association between the two GTPases by 400-fold. Using fluorescence resonance energy transfer, we demonstrate here that formation of a stable SRP x SR complex involves two distinct steps: a fast initial association between SRP and SR to form a GTP-independent early complex and then a GTP-dependent conformational rearrangement to form the stable final complex. We also found that the 4.5S SRP RNA significantly stabilizes the early GTP-independent intermediate. Furthermore, mutational analyses show that there is a strong correlation between the ability of the mutant SRP RNAs to stabilize the early intermediate and their ability to accelerate SRP x SR complex formation. We propose that the SRP RNA, by stabilizing the early intermediate, can give this transient intermediate a longer life time and therefore a higher probability to rearrange to the stable final complex. This provides a coherent model that explains how the 4.5S RNA exerts its catalytic role in SRP x SR complex assembly.
Figures
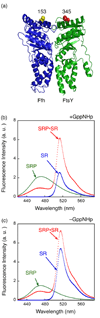
Figure 1. SRP and SR can form a complex independently of GTP
(a) Position of FRET donor (●) and acceptor (●) probes on the SRP (Ffh) and SR (FtsY) on a co-crystal structure of the Ffh•FtsY complex . (b) Fluorescence emission spectrum of SRP•SR complex in the presence of 100 µM GppNHp. 0.5 µM SRP and 2 µM SR were incubated for 10 minutes at 25 °C to form the SRP•SR complex (red). SRP- and SR-only spectra (green and blue, respectively) were obtained by incubating fluorescently labeled SRP (or SR) with unlabeled SR (or SRP). (c) Fluorescence emission spectrum of SRP•SR complex in the absence of GppNHp. 5 µM SRP and 15 µM SR were incubated at 25 °C for 10 minutes. SRP or SR-only spectra were obtained as in part (b).
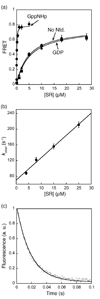
Figure 2. Stability and kinetics for formation of the GTP-independent complex
(a) Equilibrium titration of SRP•SR complex with GppNHp (●), GDP (■), and without nucleotide (▲). The data were fit to a single binding equation and gave dissociation constants of 16 nM (GppNHp), 4 µM (GDP) and 4.2 µM (no nucleotide). (b) Association kinetics of GTP-independent complex was measured as described in Methods. Values of observed rate constants were plotted against SR concentration and a linear fit of the data gave an association rate constant of 5.6 × 106 M−1 s−1. (c) Dissociation kinetics was determined in a pulse-chase experiment described in Methods. The data were fit to a single exponential equation and gave a dissociation rate constant of 60 s−1.
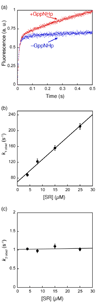
Figure 3. Formation of an SRP•SR complex in the presence of GppNHp involves two discrete steps
(a) Comparison of the time courses for complex formation in the absence (blue) and presence of 100 µM GppNHp (red). Data were obtained with 4 µM SRP and 8 µM SR. (b) The observed rate constants of the first kinetic phase during SRP-SR association in the presence of GppNHp were plotted against SR concentration. A linear fit of the data gave an association rate constant of 5.8 × 106 M−1 s−1 (k1 in Scheme I). (c) The observed rate constants of the second kinetic phase during SRP•SR association in the presence of GppNHp are independent of SR concentration. The average of these rate constants is 1.03 s−1 (k2 in Scheme I).
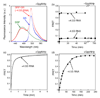
Figure 4. The GTP-independent complex is stabilized by the 4.5S RNA
(a) Spectrum of the GTP-independent complex in the absence of 4.5S RNA. The experiment setup is the same as in Fig. 1c except that the 4.5S RNA was not included. (b) Formation of the GTP-independent complex was monitored in the presence (●) and absence (■) of the 4.5S RNA. (c and d) The time course for formation of the GTP-dependent complex was monitored in the presence (c) and absence (d) of 4.5S RNA. In (c), 0.5 µM SRP and 2 µM SR were used. In (d), 2 µM Ffh and 10 µM SR were used to obtain a faster reaction rate. Note the difference in time scales in (c) and (d).
Figure 5. Tetraloop mutants in 4.5S RNA slows down the assembly rate of an active SRP•SR complex
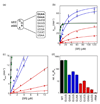
(a) List of tetraloop mutants studied in this work. GAAA and GUAA form GNRA type tetraloops (shown as bold); UUCG forms a UNRG type tetraloop (shown as italics); GUUG, GAAU, UCGA, CUUC and UGAA do not form a tetraloop (shown as normal). (b and c) Tetraloop mutants in the 4.5S RNA were classified into three classes based on the severity of defects in SRP-SR association (refer to the classification and color-coding in table 1). The GTPase reaction rate constants were measured and analyzed as described in Methods using 100 nM SRP and 100 µM GTP (wild-type (●), GUUG (■), UGAA (▲), GAAA (◆), GUCG (◆), UUCG (■), and no RNA (▲)). The initial linear portion of (b) are expanded in (c) to show the difference in _k_cat/KM of the various RNA mutants. The values of _k_cat/KM and _k_cat for each RNA are listed in Table 1. (d) Comparison of _k_cat/KM values for the various RNA mutants. Data were from Fig. 5c.
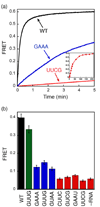
Figure 6. FRET measurement shows the deficiency in SRP-SR complex formation caused by 4.5S RNA tetraloop mutants
(a) Time course for formation of the GTP-dependent complex in the presence of different RNA mutants. The inset shows the data over a longer time course with the UUCG mutant (time scale in minutes). 0.5 µM SRP (2 µM SRP for UUCG mutant) and 2 µM SR (10 µM SR for UUCG) were used in the experiment in the presence of 100 µM GppNHp. (b) FRET measurement of the extent of formation of the GTP-independent complex with various 4.5S RNA mutants. 4 µM SRP and 16 µM SR were incubated without GppNHp.
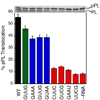
Figure 7. Tetraloop mutants impair the co-translational translocation of pre-prolactin
The translocation efficiencies were determined and analyzed as described in Methods. Top panel shows the SDS-PAGE analysis of the translocation of 35S-labeled prolactin. pPL and PL indicate the precursor and mature form of prolactin.
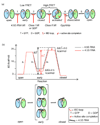
Figure 8. Multiple conformational changes during SRP-SR complex formation and activation
(a) SRP and SR GTPases form an early GTP-independent intermediate that exhibits a low FRET (step 1). In the presence of GTP, early rearranges to a more stable, closed complex that exhibits a high FRET (step 2). Additional rearrangements in the catalytic loops activate GTP hydrolysis (step 3). GTP hydrolysis drives the dissociation of the SRP•SR complex (steps 4 and 5). Each step can be blocked using specific mutants or nucleotides. 4.5S RNA tetraloop mutants block formation of the early intermediate. Class I mutants of SR or GDP blocks formation of a closed complex. Class II mutants on SRP or SR , block the rearrangement that activates GTP hydrolysis. GppNHp blocks the chemical step. (b) top panel: free energy profile for the SRP-SR interaction in the absence (black) and presence (red) of the 4.5S RNA for a standard state of 200 nM that mimics the in vivo protein concentrations in bacteria. Activation energies were calculated from the observed association and dissociation rate constants using ΔG = −RT ln(kh/kBT), where R = 1.987 cal K−1 mol−1, h = 1.58 × 10−37 kcal s−1, kB = 3.3 × 10−27 kcal K−1, and T = 298K. The relative energies of the different complexes were calculated from the observed equilibrium stabilities using ΔG = − RT ln_K_. The 4.5S RNA stabilizes the early intermediate (in bracket) by > 2.5 kcal mol−1, and the overall activation energy is subsequently lowered by ~3 kcal mol−1. ΔG≠ and ΔG≠′ defines the overall activation energy for forming the GTP-stabilized complex with and without RNA, respectively. The bottom panel depicts a physical picture of how the 4.5S RNA exerts its effect on the SRP-SR interaction as described in the text.
Scheme 1
Similar articles
- Multiple conformational switches in a GTPase complex control co-translational protein targeting.
Zhang X, Schaffitzel C, Ban N, Shan SO. Zhang X, et al. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1754-9. doi: 10.1073/pnas.0808573106. Epub 2009 Jan 27. Proc Natl Acad Sci U S A. 2009. PMID: 19174514 Free PMC article. - Multi-state targeting machinery govern the fidelity and efficiency of protein localization.
Yang M, Pang X, Han K. Yang M, et al. Adv Exp Med Biol. 2014;805:385-409. doi: 10.1007/978-3-319-02970-2_16. Adv Exp Med Biol. 2014. PMID: 24446370 - Domain rearrangement of SRP protein Ffh upon binding 4.5S RNA and the SRP receptor FtsY.
Buskiewicz I, Kubarenko A, Peske F, Rodnina MV, Wintermeyer W. Buskiewicz I, et al. RNA. 2005 Jun;11(6):947-57. doi: 10.1261/rna.7242305. RNA. 2005. PMID: 15923378 Free PMC article. - A structural step into the SRP cycle.
Wild K, Rosendal KR, Sinning I. Wild K, et al. Mol Microbiol. 2004 Jul;53(2):357-63. doi: 10.1111/j.1365-2958.2004.04139.x. Mol Microbiol. 2004. PMID: 15228518 Review. - Targeting proteins to membranes: structure of the signal recognition particle.
Egea PF, Stroud RM, Walter P. Egea PF, et al. Curr Opin Struct Biol. 2005 Apr;15(2):213-20. doi: 10.1016/j.sbi.2005.03.007. Curr Opin Struct Biol. 2005. PMID: 15837181 Review.
Cited by
- Fingerloop activates cargo delivery and unloading during cotranslational protein targeting.
Ariosa AR, Duncan SS, Saraogi I, Lu X, Brown A, Phillips GJ, Shan SO. Ariosa AR, et al. Mol Biol Cell. 2013 Jan;24(2):63-73. doi: 10.1091/mbc.E12-06-0434. Epub 2012 Nov 7. Mol Biol Cell. 2013. PMID: 23135999 Free PMC article. - Sequential activation of human signal recognition particle by the ribosome and signal sequence drives efficient protein targeting.
Lee JH, Chandrasekar S, Chung S, Hwang Fu YH, Liu D, Weiss S, Shan SO. Lee JH, et al. Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5487-E5496. doi: 10.1073/pnas.1802252115. Epub 2018 May 30. Proc Natl Acad Sci U S A. 2018. PMID: 29848629 Free PMC article. - Protein Transport Across the Bacterial Plasma Membrane by the Sec Pathway.
Smets D, Loos MS, Karamanou S, Economou A. Smets D, et al. Protein J. 2019 Jun;38(3):262-273. doi: 10.1007/s10930-019-09841-8. Protein J. 2019. PMID: 31134461 Review. - Direct visualization reveals dynamics of a transient intermediate during protein assembly.
Zhang X, Lam VQ, Mou Y, Kimura T, Chung J, Chandrasekar S, Winkler JR, Mayo SL, Shan SO. Zhang X, et al. Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6450-5. doi: 10.1073/pnas.1019051108. Epub 2011 Apr 4. Proc Natl Acad Sci U S A. 2011. PMID: 21464281 Free PMC article. - Fidelity of cotranslational protein targeting by the signal recognition particle.
Zhang X, Shan SO. Zhang X, et al. Annu Rev Biophys. 2014;43:381-408. doi: 10.1146/annurev-biophys-051013-022653. Annu Rev Biophys. 2014. PMID: 24895856 Free PMC article. Review.
References
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic-reticulum membrane. Annual Review of Cell Biology. 1994;10:87–119. - PubMed
- Matlack KES, Mothes W, Rapoport TA. Protein translocation: Tunnel vision. Cell. 1998;92:381–390. - PubMed
- Johnson AE, van Waes MA. The translocon: A dynamic gateway at the ER membrane. Annual Review of Cell and Developmental Biology. 1999;15:799–842. - PubMed
- Shan SO, Walter P. Co-translational protein targeting by the signal recognition particle. FEBS Letters. 2005;579:921–926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials