Multiple modes of engrailed regulation in the progression towards cell fate determination - PubMed (original) (raw)
Multiple modes of engrailed regulation in the progression towards cell fate determination
J Heemskerk et al. Nature. 1991.
Abstract
The engrailed gene product of Drosophila specifies the fate of a subset of cells in each segment. Our studies of engrailed regulation suggest that fate determination is an elaborate, multistep process. At the time in embryogenesis when the engrailed-dependent cell fate is probably determined, four modes of control act in an overlapping progression to govern engrailed expression. After activation by pair-rule genes, both an extracellular signal, wingless, and autoregulation are required for engrailed expression. Autoregulation graduates to wingless independence, but is transient, and is superseded by an engrailed-independent mode of maintenance.
Figures
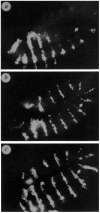
FIG. 1
Expression of en becomes _wg_-independent after 5 h. Embryos are stained with an anti-en antibody. Magnification 140×, anterior is left and ventral is down in all figures except when noted. Embryos are shifted from 18 °C to 29 °C (at different times, but age of embryos is corrected for effect of temperature and is given in hours after egg laying (AEL) as if embryos were raised at 25 °C. _a,wg_IL114ts/_wg_CX4 embryo grown at nonpermissive temperature (29 °C) from ∼3.5 to 7.5 h AEL. Expression of en is indistinguishable from that seen in a wg null embryo at this stage,. Only expression that is not under wg control persists: in the head, the first four ectodermal stripes (1–4), and a segmentally repeated subset of CNS cells. Expression has disappeared from the lateral ectoderm posterior to the fourth en stripe (for example, the normal position of stripe 7 is indicated by an arrow). In wild-type embryos at this stage, en striped expression is easily detectable (see Fig. 2b). _b,wg_IL114ts/_wg_CX4 embryo grown at nonpermissive temperature from ∼4.5 to 8 h AEL. Some ectodermal cells continue to express en, creating a partial stripe in each segment (for example, stripe 7 is indicated by an arrow). _c,wg_IL114ts/_wg_CX4 embryo grown at nonpermissive temperature from ∼5 to 8 h AEL. The en stripes are almost wild-type (for example, stripe 7 is indicated by an arrow). Some stripes are incomplete dorsally. This is the same phenotype seen when these embryos are grown exclusively at permissive temperature (18 °C). Immunocytochemistry was done as in refs 14, . The _wg_IL114ts is described in ref.13 and was obtained from the Tübingen stock collection. The _wg_CX4 is described in ref. 8.
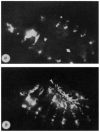
FIG. 2
Expression of en stops prematurely in en mutants. Embryos (120×) are stained with an anti-en antibody. a, Wild-type embryo, ∼4.5 h AEL. b, Wild-type embryo, ∼6.5 h AEL. c, An _en_CX1/_en_CX1 embryo ∼4.5 h AEL. The mutant cytoplasmic protein is expressed in a wild-type pattern. d, An _en_CX1/_en_CX1 embryo, ∼6.5 h AEL. The mutant protein has disappeared from the ectoderm at this stage. For example, the normal position of stripe 7 is indicated by an arrow (compare with c). Some cells of the CNS in each segment continue to express the _en_CX1 allele. The diffuse signal in other areas of the embryo is yolk autofluorescence. Loss of _en_CXI is not the result of defects in the _cis_-acting control region because this allele is expressed normally when heterozygous with wild-type en (data not shown). The _en_CX1 stock was obtained from R. Holmgren.
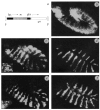
FIG. 3
Globally induced en activates the endogenous en gene in novel cells. Embryos (115×) are stained with an anti-en antibody. b–e, Embryos between 6 and 7 h AEL. a, Schematic diagram of hs–en. The en sequences consist of a 2-kb _Eco_RI–_Sna_BI fragment of the en cDNA clone c-2.4 (ref. 32). Transcription of en sequences (stippled box) is driven by the hsp70 heat-shock promoter (hsp), contained in a 1.3-kb Sph_l/Pst_l fragment from pUCHSP 70 (provided by E. Gavis). The polyadenylation sequences (pA+) are from SV40. For transformation, the above sequences are inserted into the pCaSpeR vector containing P element ends (ovals) and the white gene (w+) as a selectable marker. b, An hs–_en embryo, heat shocked at ∼6 h, then immediately fixed and stained. The en expression induced by heat shock is detectable in all cells. c, An hs–_en embryo, heat shocked at ∼3 h AEL, aged 3.5 h, then fixed and stained. The en stripes are abnormally broad in the ventral and lateral regions compared with wild type (compare distance between arrowheads in c and d). Inset, 326× magnification of part of one stripe showing that the en antigen is confined to the nucleus. d, Wild-type embryo, showing the normal pattern of en expression. e, An en_CX1/+;hs–_en embryo, heat shocked at ∼3 h AEL, aged 3.5 h, then fixed and stained. The pattern of cytoplasmic _en_CXI antigen is similar to the pattern of en in c. Inset is a 326× magnification of part of one stripe showing that the cytoplasmic antigen, characteristic of en_CX1, is produced in novel cells after heat shock. Both the wild-type nuclear and mutant cytoplasmic en antigens are expressed in these cells, although the nuclear signal is weak relative to the cytoplasmic signal. f, An hs–_en embryo, heat shocked at ∼5 h then fixed after 3 h recovery at 25 °C. Striped en staining is nearly wild type. There are isolated cells in the anterior compartment that express en (small arrowheads), as well as staining in the amnioserosa (large arrowhead), neither of which is seen in wild type. METHODS. Transgenic lines were established using the p-wings clipped helper and Df(1)w_67c2,y as host. For heat-shock experiments, embryos were collected and aged at 25 °C on grape agar plates. At the appropriate stage, plates were put in moist chambers at 37 °C for 35 min. They were then returned to 25 °C for recovery. The novel expression pattern was detectable as soon as the en expressed from the heat-shock promoter decayed to background levels, ∼45 min after heat shock. Similar results were seen with three different hs–_en lines, one on chromosome III and two on chromosome II.
FIG. 4
Decay of wg RNA after heat shock of hs–en embryos. Embryos (125×) are hybridized with a digoxygenin-labelled wg probe. a, Wild-type embryo, ∼5 h AEL, showing the normal pattern of wg RNA expression. b, An hs–en embryo, heat shocked at ∼3 h, then allowed to recover at 25 °C for 1 h. Although the patches of wg expression in the head are of normal intensity (arrowhead, compare with a), the striped expression in the ectoderm is dramatically reduced (for comparison, arrow points to the stripe that corresponds to the arrow in a). Signal persists mostly in the ventral ectoderm. c, An hs–en embryo, heat shocked at ∼3 h and allowed to recover for 2 h at 25 °C. There is normal wg staining in the head (arrowhead), but all striped ectodermal RNA has decayed (arrow shows the normal position of the stripe shown by arrows in a and b). METHODS. Embryos were fixed and probed for wg RNA as in ref.30. The wg probe was made from a 1.3-kb genomic Eco_RI–_Hind_III fragment containing the fourth and fifth exons (provided by N. Baker). In a collection of embryos from parents that were heterozygous for the hs–_en insert, only 3/4 showed premature loss of wg RNA after heat shock, consistent with the loss resulting from hs–en, due to either heat-shock-produced en or stable ectopic en.
FIG. 5
Late autoregulation is wg independent. Embryos (140×) are stained with an anti-en antibody. a, A _wg_CX4/_wg_CX4 embryo, ∼8 h AEL. There is no striped en expression in the ectoderm posterior to the third en stripe. For example, the arrow points to the normal position of en stripe 7. The repeated staining in the ventral region is in the CNS. b, A wg_CX4/wg_CX4;hs–_en embryo, ∼8 h AEL. Embryo was heat shocked at ∼5 h then fixed after 3 h recovery at 25 °C. There are partial en stripes in positions that have no signal in a wg mutant without heat shock. For example, the arrow points to the partially rescued stripe 7. The staining phenotype varied. The embryo shown is representative of about half of the wg_CX4/wg_CX4;hs–_en embryos heat shocked at this stage. The others had relatively fewer ectodermal cells staining. As with wild-type hs–_en, there is staining in cells of the amnioserosa (arrowhead). METHODS. Embryos were derived from parents that were heterozygous for wg_CX4 on chromosome II and hs–_en on chromosome III. Thus, 3 out of 16 of the embryos are homozygous for the wg mutation and carry at least one copy of hs–_en. Consistent with this, 3 out of 16 of the embryos show the phenotype we interpret as wg_CX4/wg_CX4;hs–_en, that is dramatically fewer than the wild-type number of en cells staining, due to lack of wg, and en staining in the amnioserosa, due to activity of the hs–_en insert.
FIG. 6
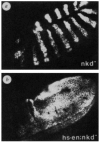
Autoregulation is repressed by nkd. Embryos (150×) are stained with an anti-en antibody. a, A _nkd_7E/_nkd_7E embryo, ∼5 h AEL, showing abnormally broad en stripes compared with wild type. The broadening is due to abnormal en expression in cells posterior to the normal en stripe in nkd mutants (S.D., unpublished observation). b, A _nkd_7E/nkd_7E;hs–_en embryo heat shocked at ∼3 h and fixed after recovering for 2 h at 25 °C. Nearly all ectodermal cells stain except areas of the head and telson. In addition, some isolated areas in the ventral–lateral ectoderm do not express en. These cells vary somewhat in number and position from embryo to embryo, and thus do not appear to be cells of one specific type. METHODS. The nkd_7E/nkd_7E;hs–_en embryos came from parents that were heterozygous for nkd_7E on chromosome III and homozygous for hs–_en on chromosome II. Thus, 1/4 of the embryos were homozygous for nkd and carried hs–_en, and 1/4 of the embryos showed global staining in the ectoderm after recovery from heat shock. The _nkd_7E stock was obtained from the Tübingen stock collection.
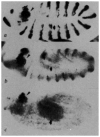
FIG. 7
A fourth mode of en control follows autoregulation. a, An hs–_en;en_CXI/_en_CXI embryo (110×), heat shocked at ∼5 h, aged 3 h, then fixed and stained with an anti-en antibody. The cytoplasmic _en_CXI protein is expressed in partial stripes in each segment. _en_CXI mutants normally show no expression by this stage. The number of cells that are rescued by heat shock varies, depending in part on the age at heat shock. This embryo is representative for a heat shock done at 5 h. Most rescued cells lie in the lateral and dorsal ectoderm and are a subset of the cells that initially express _en_CXI in early embryos (see text). b, An en–lac_-E;hs–_en embryo (75×, ventral view, anterior left) heat shocked at ∼3 h, aged 2 h, then fixed and hybridized with a digoxygenin-labelled lac-Z probe. Stripes of lac-Z RNA are abnormally broad compared with non-heat shocked control embryos (compare distance between arrowheads flanking the stripes in b and c). c, An _en–lac_-E embryo (75×, ventral view, anterior left), fixed at ∼5 h and hybridized with a digoxygenin-labelled lac-Z probe. The en–lac_-E contains 2.4 kb of en promoter/upstream sequences and the en first intron driving lac-Z expression. Stripes of lac-Z RNA correspond to the position of en expression, although the level of lac-Z expression from this construct varies among the stripes. METHODS. a, Embryos were derived from parents that were heterozygous for en_CXI on chromosome II and hs–_en on chromosome III. Thus, 3/16 of the embryos are homozygous for the en mutation and carry at least one copy of hs–_en. Consistently, 3/16 of the embryos show the phenotype we interpret as _en_CXI/en_CXI;hs–_en, that is cytoplasmic stain in ectodermal stripes with fewer than the normal number of cells staining in each stripe. b, c, Embryos were fixed and probed for lac-Z RNA as in ref. 30. The _en–lac_-E stock was obtained from J. Kassis.
FIG. 8
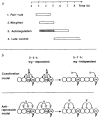
Integrating four modes of en regulation during embryogenesis. a, Approximate periods of action of the four modes of en regulation and their temporal overlap. The time line indicates hours AEL. 1, The start of the pair-rule period is defined as the time of the earliest appearance of en expression,. The end is defined as the time when en expression stops in wg mutants, presumably a condition in which only pair-rule function activates en. 2, The start of wg influence is defined as the earliest time that heat shock stably induces _wg_-dependent ectopic en expression. The end of the wg period is defined as the latest time at which raising the temperature of _wg_ts embryos has an effect on en expression. 3, The start of autoregulatory influence is taken as the earliest time that heat shock stably induces ectopic en. The end of the period during which en autoregulation operates is inferred from two observations: first, that _en_-responsive sequences cannot drive lac-Z expression past this time, and second, that heat shock does not activate expression in en mutants past this time. Stippling indicates the time during which autoregulation requires wg input. 4, The fourth mode of regulation (late control) begins when heat shock first results in stable expression in en mutants. It is not known whether this regulatory phase is responsible for maintaining en indefinitely or whether still later transitions occur. b, Two models to account for the onset of _wg_-independent en autoregulation. Cells spanning two segment primordia are schematized as eight open circles. Labelled cells represent those that normally express en or wg. Coactivation model: cells directly exposed to the wg coactivation signal are competent to autoregulate during the _wg_-dependent period. Autoregulation is allowed in whichever of these cells en is activated, either by pair-rule gene activity, or by heat shock. Expression of en becomes _wg_-independent when another coactivator (A) takes over. This new coactivation is confined to posterior compartment cells, and en expression is thereby restricted to the posterior compartment after late heat shock. Antirepression model: wg prevents a general repressor of en (R) from acting on wg cells and their neighbours during the _wg_-dependent period. Autoregulation is allowed in whichever of these cells en is activated, either by pair-rule gene activity, or by heat shock. Later, the wg requirement is relieved because late repression (R′) is confined to the anterior compartment. Autoregulation of en can now act in the normal _en_-expressing cells in the absence of the wg signal. Late heat shock cannot overcome the late repression in the anterior compartment and so does not induce ectopic autoregulation. It should be noted that, although we suggest that ectopically induced en expression occurs in the _wg_-expressing cells, we have not yet experimentally identified which cells induce en after heat shock. Additionally, wg is still expressed at late stages, but has not been indicated in the schematic because it is dispensible for en expression.
Similar articles
- Differential requirements for segment polarity genes in wingless signaling.
Noordermeer J, Klingensmith J, Nusse R. Noordermeer J, et al. Mech Dev. 1995 Jun;51(2-3):145-55. doi: 10.1016/0925-4773(95)00348-7. Mech Dev. 1995. PMID: 7547463 - Distinguishable functions for engrailed and invected in anterior-posterior patterning in the Drosophila wing.
Simmonds AJ, Brook WJ, Cohen SM, Bell JB. Simmonds AJ, et al. Nature. 1995 Aug 3;376(6539):424-7. doi: 10.1038/376424a0. Nature. 1995. PMID: 7630417 - Three distinct roles for the engrailed gene in Drosophila wing development.
Hidalgo A. Hidalgo A. Curr Biol. 1994 Dec 1;4(12):1087-98. doi: 10.1016/s0960-9822(00)00247-5. Curr Biol. 1994. PMID: 7704572 - Cell patterning in the Drosophila segment: engrailed and wingless antigen distributions in segment polarity mutant embryos.
van den Heuvel M, Klingensmith J, Perrimon N, Nusse R. van den Heuvel M, et al. Dev Suppl. 1993:105-14. Dev Suppl. 1993. PMID: 8049466 Review. - Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis.
DiNardo S, Sher E, Heemskerk-Jongens J, Kassis JA, O'Farrell PH. DiNardo S, et al. Nature. 1988 Apr 14;332(6165):604-9. doi: 10.1038/332604a0. Nature. 1988. PMID: 3282172 Free PMC article. Review.
Cited by
- Maintenance of the engrailed expression pattern by Polycomb group genes in Drosophila.
Moazed D, O'Farrell PH. Moazed D, et al. Development. 1992 Nov;116(3):805-10. doi: 10.1242/dev.116.3.805. Development. 1992. PMID: 1363229 Free PMC article. - Drawing lines in the sand: even skipped et al. and parasegment boundaries.
Jaynes JB, Fujioka M. Jaynes JB, et al. Dev Biol. 2004 May 15;269(2):609-22. doi: 10.1016/j.ydbio.2004.03.001. Dev Biol. 2004. PMID: 15110723 Free PMC article. - Separable regulatory elements mediate the establishment and maintenance of cell states by the Drosophila segment-polarity gene gooseberry.
Li X, Gutjahr T, Noll M. Li X, et al. EMBO J. 1993 Apr;12(4):1427-36. doi: 10.1002/j.1460-2075.1993.tb05786.x. EMBO J. 1993. PMID: 8096813 Free PMC article. - The topology of the regulatory interactions predicts the expression pattern of the segment polarity genes in Drosophila melanogaster.
Albert R, Othmer HG. Albert R, et al. J Theor Biol. 2003 Jul 7;223(1):1-18. doi: 10.1016/s0022-5193(03)00035-3. J Theor Biol. 2003. PMID: 12782112 Free PMC article. - The Drosophila engrailed and invected genes: partners in regulation, expression and function.
Gustavson E, Goldsborough AS, Ali Z, Kornberg TB. Gustavson E, et al. Genetics. 1996 Mar;142(3):893-906. doi: 10.1093/genetics/142.3.893. Genetics. 1996. PMID: 8849895 Free PMC article.
References
- Slack JMW. Egg to Embryo: Determinative Events in Early Development. Cambridge University Press; Cambridge: 1983.
- Garcia-Bellido A. CIBA Fdn Symp. 1975;29:161–182. - PubMed
- Lawrence PA, Morata G. Devl Biol. 1976;50:321–337. - PubMed
- Kornberg T, Siden I, O'Farrell P, Simon M. Cell. 1985;40:45–53. - PubMed
- Garcia-Bellido A, Ripoll P, Morata G. Nature. 1973;245:251–253. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases