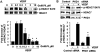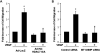VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis - PubMed (original) (raw)
VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis
Chang Hoon Ha et al. Arterioscler Thromb Vasc Biol. 2008 Oct.
Abstract
Objective: Histone acetylation/deacetylation plays an important role in the control of gene expression, tissue growth, and development. In particular, histone deacetylases 7 (HDAC7), a member of class IIa HDACs, is crucial in maintaining vascular integrity. However, whether HDAC7 is involved in the processes of vascular endothelial signaling and angiogenesis remains unclear. Here, we investigated the role of HDAC7 in vascular endothelial growth factor (VEGF) signaling and angiogenesis.
Methods and results: We show for the first time that VEGF stimulated phosphorylation of HDAC7 at the sites of Ser178, Ser344, and Ser479 in a dose- and time-dependent manner, which leads to the cytoplasmic accumulation of HDAC7. Using pharmacological inhibitors, siRNA, and adenoviruses carrying dominant-negative mutants, we found that phospholipase Cgamma/protein kinase C/protein kinase D1 (PKD1)-dependent signal pathway mediated HDAC7 phosphorylation and cytoplasmic accumulation by VEGF. Infection of ECs with adenoviruses encoding a mutant of HDAC7 specifically deficient in PKD1-dependent phosphorylation inhibited VEGF-induced angiogenic gene expression, including matrix metalloproteinases MT1-matrix metalloproteinase (MMP) and MMP10. Moreover, HDAC7 and its targeting genes were involved in VEGF-stimulated endothelial cell migration, tube formation, and microvessel sprouting.
Conclusions: Our results demonstrate that VEGF stimulates PKD1-dependent HDAC7 phosphorylation and cytoplasmic accumulation in endothelial cells modulating gene expression and angiogenesis.
Figures
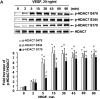
Figure 1. VEGF stimulates HDAC7 phosphorylation in endothelial cells
(A) BAECs were exposed to VEGF (20 ng/ml) for various times as indicated. (B) BAECs were exposed to VEGF for 15 min with the indicated concentrations. HDAC7 phosphorylation and expression in cell lysates were determined as described in Materials and Methods. Representative immunoblots and quantitative data of HAC7 phosphorylation normalized with the level of HDAC7 were shown (n=4). *, # and &, p < 0.05 in comparison to value from control group without VEGF treatment (0 min).
Figure 1. VEGF stimulates HDAC7 phosphorylation in endothelial cells
(A) BAECs were exposed to VEGF (20 ng/ml) for various times as indicated. (B) BAECs were exposed to VEGF for 15 min with the indicated concentrations. HDAC7 phosphorylation and expression in cell lysates were determined as described in Materials and Methods. Representative immunoblots and quantitative data of HAC7 phosphorylation normalized with the level of HDAC7 were shown (n=4). *, # and &, p < 0.05 in comparison to value from control group without VEGF treatment (0 min).
Figure 2. PKD1 mediates HDAC7 phosphorylation by VEGF
(A) BAECs were pretreated with the potent PKD inhibitor Go6976 at indicated concentration for 30 min, and then exposed to VEGF (20 ng/ml) for 15 min. (B) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 48 h, and then exposed to VEGF for 15 min. (C) BAECs were infected with adenoviruses encoding GFP (Ad-GFP, control), or Ad-GFP-PKD1-KN at 100 MOI for 24 h, and then exposed to VEGF for 15 min. HDAC7 phosphorylation and expression in cell lysates were determined. PKD1 expression levels were also analyzed to confirm the knockdown effect of PKD1 siRNA (B) and overexpression of GFP-PKD1-KN in ECs (C). Representative immunoblots and quantitative data were shown (n=3).
Figure 2. PKD1 mediates HDAC7 phosphorylation by VEGF
(A) BAECs were pretreated with the potent PKD inhibitor Go6976 at indicated concentration for 30 min, and then exposed to VEGF (20 ng/ml) for 15 min. (B) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 48 h, and then exposed to VEGF for 15 min. (C) BAECs were infected with adenoviruses encoding GFP (Ad-GFP, control), or Ad-GFP-PKD1-KN at 100 MOI for 24 h, and then exposed to VEGF for 15 min. HDAC7 phosphorylation and expression in cell lysates were determined. PKD1 expression levels were also analyzed to confirm the knockdown effect of PKD1 siRNA (B) and overexpression of GFP-PKD1-KN in ECs (C). Representative immunoblots and quantitative data were shown (n=3).
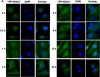
Figure 3. VEGF promotes cytoplasmic accumulation of HDAC7 through PKD1-dependent phosphorylation
(A-B) BAECs were infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, and then exposed to VEGF (20 ng/ml) for various times as indicated (A); or then pretreated with PKD inhibitor Go6976 (1 μM) for 30 min followed by the exposure of VEGF for 3 h (B). (C) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 24 h, and then infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, followed by the exposure of VEGF (20 ng/ml) for 3 h. (D) BAECs were infected with Ad-YFP-HDAC7-WT or Ad- YFP-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF for 3 h. (E) BAECs were infected with Ad-HA-HDAC7-WT or Ad- HA-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF (20 ng/ml) for 3 h. The cells were fixed and YFP-HDAC7 or HA-HDAC7 distribution in the cell was analyzed by fluorescence microscopy. Representative images (magnification, x40 for (A) or x60 for (B-E)) were shown (n=3). Nuclei stained with DAPI (A) were also analyzed by fluorescence microscopy.
Figure 3. VEGF promotes cytoplasmic accumulation of HDAC7 through PKD1-dependent phosphorylation
(A-B) BAECs were infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, and then exposed to VEGF (20 ng/ml) for various times as indicated (A); or then pretreated with PKD inhibitor Go6976 (1 μM) for 30 min followed by the exposure of VEGF for 3 h (B). (C) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 24 h, and then infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, followed by the exposure of VEGF (20 ng/ml) for 3 h. (D) BAECs were infected with Ad-YFP-HDAC7-WT or Ad- YFP-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF for 3 h. (E) BAECs were infected with Ad-HA-HDAC7-WT or Ad- HA-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF (20 ng/ml) for 3 h. The cells were fixed and YFP-HDAC7 or HA-HDAC7 distribution in the cell was analyzed by fluorescence microscopy. Representative images (magnification, x40 for (A) or x60 for (B-E)) were shown (n=3). Nuclei stained with DAPI (A) were also analyzed by fluorescence microscopy.
Figure 3. VEGF promotes cytoplasmic accumulation of HDAC7 through PKD1-dependent phosphorylation
(A-B) BAECs were infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, and then exposed to VEGF (20 ng/ml) for various times as indicated (A); or then pretreated with PKD inhibitor Go6976 (1 μM) for 30 min followed by the exposure of VEGF for 3 h (B). (C) HUVECs were transfected with scrambled siRNA (100 nM, control) or PKD1 siRNA (100 nM) for 24 h, and then infected with Ad-YFP-HDAC7 at 50 MOI for 24 h, followed by the exposure of VEGF (20 ng/ml) for 3 h. (D) BAECs were infected with Ad-YFP-HDAC7-WT or Ad- YFP-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF for 3 h. (E) BAECs were infected with Ad-HA-HDAC7-WT or Ad- HA-HDAC7-S/A at 50 MOI for 24 h, and then stimulated with VEGF (20 ng/ml) for 3 h. The cells were fixed and YFP-HDAC7 or HA-HDAC7 distribution in the cell was analyzed by fluorescence microscopy. Representative images (magnification, x40 for (A) or x60 for (B-E)) were shown (n=3). Nuclei stained with DAPI (A) were also analyzed by fluorescence microscopy.
Figure 4. PKD1-dependent HDAC7 phosphorylation modulates VEGF-induced MMP10 and MT1-MMP expression in endothelial cells
(A) HUVECs were stimulated with VEGF (20 ng/ml) for various times as indicated. (B) HUVECs were infected with Ad-GFP (control), Ad-YFP-HDAC7-S/A or GFP-PKD1-KN (100 MOI) for 24 h, and then stimulated with VEGF (20 ng/ml) for 1 h. The mRNA was extracted from the cell lysates, and RT-PCR with the primers of MMP1, MT1-MMP, and GADPH (internal control) were performed as described in Materials and Methods. The representative images and quantitative data were shown (n=4).
Figure 5. HDAC7 and MT1-MMP are involved in VEGF-induced endothelial cell migration and tube formation
(A) BAECs were infected with Ad-LacZ (control), or Ad-HA-HDAC7-S/A and then measured for cell migration in response to VEGF in wound closure assay (n=4). (B) HUVECs were transfected with control siRNA or human MT1-MMP1 siRNA and then measured for cell migration in response to VEGF in wound closure assay. The representative images and quantitative data were shown (n=4). (C) HUVECs infected with Ad-LacZ (control), or Ad-HA-HDAC7-S/A were cultured in Matrigel and measured for in vitro angiogenesis in response to VEGF with tube formation assay (n=4). (D) HUVECs transfected with control siRNA or human MT1-MMP1 siRNA were cultured in Matrigel and measured for in vitro angiogenesis in response to VEGF with tube formation assay. The length of tube-like structure were quantified and shown. *, p < 0.05 in comparison to value from control; #, p < 0.05 vs. that from the group treated with VEGF along with Ad-lacZ or control siRNA.
Figure 5. HDAC7 and MT1-MMP are involved in VEGF-induced endothelial cell migration and tube formation
(A) BAECs were infected with Ad-LacZ (control), or Ad-HA-HDAC7-S/A and then measured for cell migration in response to VEGF in wound closure assay (n=4). (B) HUVECs were transfected with control siRNA or human MT1-MMP1 siRNA and then measured for cell migration in response to VEGF in wound closure assay. The representative images and quantitative data were shown (n=4). (C) HUVECs infected with Ad-LacZ (control), or Ad-HA-HDAC7-S/A were cultured in Matrigel and measured for in vitro angiogenesis in response to VEGF with tube formation assay (n=4). (D) HUVECs transfected with control siRNA or human MT1-MMP1 siRNA were cultured in Matrigel and measured for in vitro angiogenesis in response to VEGF with tube formation assay. The length of tube-like structure were quantified and shown. *, p < 0.05 in comparison to value from control; #, p < 0.05 vs. that from the group treated with VEGF along with Ad-lacZ or control siRNA.
Figure 6. PKD1-dependent HDAC7 phosphorylation is required for VEGF-induced microvessel sprouting in mouse aorta ring assay
(A) Mouse aorta rings were isolated form mice and infected Ad-LacZ (control), Ad-HA-HDAC7-S/A or Ad-GFP-PKD1-KN, and then the aorta ring assay for ex vivo angiogenesis was performed. The representative images were shown (n=4). (B) Schema for potential role and pathways of PKD1 and HDAC7 for VEGF-induced angiogenesis (see the text in detail).
Figure 6. PKD1-dependent HDAC7 phosphorylation is required for VEGF-induced microvessel sprouting in mouse aorta ring assay
(A) Mouse aorta rings were isolated form mice and infected Ad-LacZ (control), Ad-HA-HDAC7-S/A or Ad-GFP-PKD1-KN, and then the aorta ring assay for ex vivo angiogenesis was performed. The representative images were shown (n=4). (B) Schema for potential role and pathways of PKD1 and HDAC7 for VEGF-induced angiogenesis (see the text in detail).
Comment in
- A new kid on the block: PKD1: a promising target for antiangiogenic therapy?
Altschmied J, Haendeler J. Altschmied J, et al. Arterioscler Thromb Vasc Biol. 2008 Oct;28(10):1689-90. doi: 10.1161/ATVBAHA.108.174250. Arterioscler Thromb Vasc Biol. 2008. PMID: 18799796 No abstract available.
Similar articles
- Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis.
Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Ha CH, et al. J Biol Chem. 2008 May 23;283(21):14590-9. doi: 10.1074/jbc.M800264200. Epub 2008 Mar 10. J Biol Chem. 2008. PMID: 18332134 Free PMC article. - A new kid on the block: PKD1: a promising target for antiangiogenic therapy?
Altschmied J, Haendeler J. Altschmied J, et al. Arterioscler Thromb Vasc Biol. 2008 Oct;28(10):1689-90. doi: 10.1161/ATVBAHA.108.174250. Arterioscler Thromb Vasc Biol. 2008. PMID: 18799796 No abstract available. - VEGF-PKD1-HDAC7 signaling promotes endothelial progenitor cell migration and tube formation.
Yu D, Chen W, Ren J, Zhang T, Yang K, Wu G, Liu H. Yu D, et al. Microvasc Res. 2014 Jan;91:66-72. doi: 10.1016/j.mvr.2013.10.006. Epub 2013 Nov 2. Microvasc Res. 2014. PMID: 24189120 - Protein kinase D1, a new molecular player in VEGF signaling and angiogenesis.
Ha CH, Jin ZG. Ha CH, et al. Mol Cells. 2009 Jul 31;28(1):1-5. doi: 10.1007/s10059-009-0109-9. Epub 2009 Jul 20. Mol Cells. 2009. PMID: 19655095 Free PMC article. Review. - HDAC7: a promising target in cancer.
Liu C, Zheng D, Pu X, Li S. Liu C, et al. Front Oncol. 2024 Feb 28;14:1327933. doi: 10.3389/fonc.2024.1327933. eCollection 2024. Front Oncol. 2024. PMID: 38487728 Free PMC article. Review.
Cited by
- Angiopoietin-1 and vascular endothelial growth factor regulation of leukocyte adhesion to endothelial cells: role of nuclear receptor-77.
Ismail H, Mofarrahi M, Echavarria R, Harel S, Verdin E, Lim HW, Jin ZG, Sun J, Zeng H, Hussain SN. Ismail H, et al. Arterioscler Thromb Vasc Biol. 2012 Jul;32(7):1707-16. doi: 10.1161/ATVBAHA.112.251546. Epub 2012 May 24. Arterioscler Thromb Vasc Biol. 2012. PMID: 22628435 Free PMC article. - Increased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusion.
Ehlken C, Grundel B, Michels D, Junker B, Stahl A, Schlunck G, Hansen LL, Feltgen N, Martin G, Agostini HT, Pielen A. Ehlken C, et al. PLoS One. 2015 May 15;10(5):e0126859. doi: 10.1371/journal.pone.0126859. eCollection 2015. PLoS One. 2015. PMID: 25978399 Free PMC article. - Novel aspects of corneal angiogenic and lymphangiogenic privilege.
Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH. Ellenberg D, et al. Prog Retin Eye Res. 2010 May;29(3):208-48. doi: 10.1016/j.preteyeres.2010.01.002. Epub 2010 Jan 25. Prog Retin Eye Res. 2010. PMID: 20100589 Free PMC article. Review. - Emerging roles of protein kinase D1 in cancer.
Sundram V, Chauhan SC, Jaggi M. Sundram V, et al. Mol Cancer Res. 2011 Aug;9(8):985-96. doi: 10.1158/1541-7786.MCR-10-0365. Epub 2011 Jun 16. Mol Cancer Res. 2011. PMID: 21680539 Free PMC article. Review.
References
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - PubMed
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. - PubMed
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. - PubMed
- Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK062985/DK/NIDDK NIH HHS/United States
- R01 HL-080611/HL/NHLBI NIH HHS/United States
- R01 DK62985/DK/NIDDK NIH HHS/United States
- R01 HL080611/HL/NHLBI NIH HHS/United States
- R01 HL080611-01/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases