Animal models of acute lung injury - PubMed (original) (raw)
Review
Animal models of acute lung injury
Gustavo Matute-Bello et al. Am J Physiol Lung Cell Mol Physiol. 2008 Sep.
Abstract
Acute lung injury in humans is characterized histopathologically by neutrophilic alveolitis, injury of the alveolar epithelium and endothelium, hyaline membrane formation, and microvascular thrombi. Different animal models of experimental lung injury have been used to investigate mechanisms of lung injury. Most are based on reproducing in animals known risk factors for ARDS, such as sepsis, lipid embolism secondary to bone fracture, acid aspiration, ischemia-reperfusion of pulmonary or distal vascular beds, and other clinical risks. However, none of these models fully reproduces the features of human lung injury. The goal of this review is to summarize the strengths and weaknesses of existing models of lung injury. We review the specific features of human ARDS that should be modeled in experimental lung injury and then discuss specific characteristics of animal species that may affect the pulmonary host response to noxious stimuli. We emphasize those models of lung injury that are based on reproducing risk factors for human ARDS in animals and discuss the advantages and disadvantages of each model and the extent to which each model reproduces human ARDS. The present review will help guide investigators in the design and interpretation of animal studies of acute lung injury.
Figures
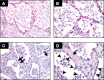
Fig. 1.
Human ARDS. Photomicrographs from the lungs of 2 different patients with ARDS stained with H&E. The alveolar spaces are filled with a mixed mononuclear/neutrophilic infiltrate, the alveolar walls are thickened, and the septae are edematous. Note the presence of cellular debris and proteinaceous material in the air spaces (A, magnification ×200; B, ×400). In later stages, there is a fibroproliferative response with collagen deposition in the alveolar walls (arrows). Note that the alveolar epithelium has been replaced with cuboidal cells (arrowheads). Magnification in C, ×200; D, ×400.
Fig. 2.
Oleic acid model. Rabbit lungs 6 h after the onset of intravenous infusion of saline (A) or 0.1 ml·kg−1·h−1 oleic acid over 2 h (B). Note the presence of hemorrhage, hyaline membrane formation, and inflammatory infiltrates in the lungs of the rabbit treated with oleic acid. Both rabbits were mechanically ventilated for the duration of the experiment (F
i
O2 = 0.8, respiratory rate = 30 bpm, PEEP = 2 cmH2O, tidal volume = 10 cc/kg). [From Furue et al. (74).]
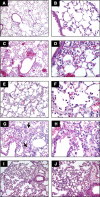
Fig. 3.
Comparison of selected models of acute lung injury (ALI). A and B: normal mouse lungs. The alveolar walls are very thin, and the majority of the alveoli contain no cells (magnification in A, ×100; B, ×400). C and D: lungs from a mouse euthanized 18 h after intratracheal instillation of 5 ng/g LPS. Note the patchy nature of the injury (C, ×100) and the presence of inflammatory infiltrates and vascular congestion (D, ×400). E and F: lungs from a rabbit euthanized 2 h after exposure to mechanical ventilation with Tv = 25 cc/kg, PEEP = 2.5 cmH2O, F
i
O2 = 0.5, and RR = 20 bpm. Note the presence of intra-alveolar neutrophilic infiltrates and the deposition of hyaline membranes (E, ×200; F, ×630). G and H: lungs from a mouse euthanized 21 days after the administration of intratracheal bleomycin. Note the presence of fibrotic areas (arrows) (G, ×200; H, ×400). I and J: lungs from a mouse euthanized 12 h after aerosolization of Escherichia coli, 1 × 108 cfu/ml. Note diffuse thickening of the alveolar spaces and intra-alveolar neutrophilic infiltrates (I, ×200; J, ×400). Hematoxylin and eosin.
Fig. 4.
Acid aspiration model. Lung tissue sections from a normal mouse (left) and a mouse euthanized 2 h after intratracheal instillation of 1 M HCl, 2 μl/g (pH = 1.5) (right). Note the presence of intra-alveolar proteinaceous deposits (arrow). [From Zarbock et al. (251).]
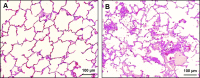
Fig. 5.
Cecal ligation and puncture (CLP). Lungs from mice following sham surgery (left) or from mice subjected to 90 min of hemorrhagic shock (MAP = 30 mmHg) followed 24 h later by CLP (right). The lungs were stained for neutrophil-specific esterase (red). [From Lomas-Neira et al. (128).]
Comment in
- Hyperoxia and acute lung injury.
Fisher AB, Beers MF. Fisher AB, et al. Am J Physiol Lung Cell Mol Physiol. 2008 Dec;295(6):L1066; author reply L1067. doi: 10.1152/ajplung.90486.2008. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 19047485 Free PMC article. No abstract available.
Similar articles
- Hyperoxia and acute lung injury.
Fisher AB, Beers MF. Fisher AB, et al. Am J Physiol Lung Cell Mol Physiol. 2008 Dec;295(6):L1066; author reply L1067. doi: 10.1152/ajplung.90486.2008. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 19047485 Free PMC article. No abstract available. - Open Tracheostomy Gastric Acid Aspiration Murine Model of Acute Lung Injury Results in Maximal Acute Nonlethal Lung Injury.
Alluri R, Kutscher HL, Mullan BA, Davidson BA, Knight PR. Alluri R, et al. J Vis Exp. 2017 Feb 26;(120):54700. doi: 10.3791/54700. J Vis Exp. 2017. PMID: 28287530 Free PMC article. - The HDL from septic-ARDS patients with composition changes exacerbates pulmonary endothelial dysfunction and acute lung injury induced by cecal ligation and puncture (CLP) in mice.
Yang L, Liu S, Han S, Hu Y, Wu Z, Shi X, Pang B, Ma Y, Jin J. Yang L, et al. Respir Res. 2020 Nov 4;21(1):293. doi: 10.1186/s12931-020-01553-3. Respir Res. 2020. PMID: 33148285 Free PMC article. - Overview of the pathology of three widely used animal models of acute lung injury.
Wang HM, Bodenstein M, Markstaller K. Wang HM, et al. Eur Surg Res. 2008;40(4):305-16. doi: 10.1159/000121471. Epub 2008 Mar 19. Eur Surg Res. 2008. PMID: 18349543 Review. - Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation.
Gonçalves-de-Albuquerque CF, Silva AR, Burth P, Castro-Faria MV, Castro-Faria-Neto HC. Gonçalves-de-Albuquerque CF, et al. Mediators Inflamm. 2015;2015:260465. doi: 10.1155/2015/260465. Epub 2015 Nov 12. Mediators Inflamm. 2015. PMID: 26640323 Free PMC article. Review.
Cited by
- Prime-O-glucosylcimifugin attenuates lipopolysaccharide-induced acute lung injury in mice.
Chen N, Wu Q, Chi G, Soromou LW, Hou J, Deng Y, Feng H. Chen N, et al. Int Immunopharmacol. 2013 Jun;16(2):139-47. doi: 10.1016/j.intimp.2013.04.014. Epub 2013 Apr 24. Int Immunopharmacol. 2013. PMID: 23623941 Free PMC article. - Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock.
Li Y, Alam HB. Li Y, et al. Adv Exp Med Biol. 2012;710:107-33. doi: 10.1007/978-1-4419-5638-5_11. Adv Exp Med Biol. 2012. PMID: 22127890 Free PMC article. - Effect of insulin-like growth factor blockade on hyperoxia-induced lung injury.
Kim TH, Chow YH, Gill SE, Schnapp LM. Kim TH, et al. Am J Respir Cell Mol Biol. 2012 Sep;47(3):372-8. doi: 10.1165/rcmb.2012-0085OC. Epub 2012 Apr 5. Am J Respir Cell Mol Biol. 2012. PMID: 22493012 Free PMC article. - Ex Vivo Perfusion With Methylprednisolone Attenuates Brain Death-induced Lung Injury in Rats.
van Zanden JE, Leuvenink HGD, Verschuuren EAM, Veldhuis ZJ, Ottens PJ, Erasmus ME, Hottenrott MC. van Zanden JE, et al. Transplant Direct. 2021 Mar 16;7(4):e682. doi: 10.1097/TXD.0000000000001141. eCollection 2021 Apr. Transplant Direct. 2021. PMID: 33748411 Free PMC article. - Evaluation of the BDCA2-DTR Transgenic Mouse Model in Chronic and Acute Inflammation.
Mandl M, Drechsler M, Jansen Y, Neideck C, Noels H, Faussner A, Soehnlein O, Weber C, Döring Y. Mandl M, et al. PLoS One. 2015 Aug 7;10(8):e0134176. doi: 10.1371/journal.pone.0134176. eCollection 2015. PLoS One. 2015. PMID: 26252890 Free PMC article.
References
- Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol : L533–L542, 2004. - PubMed
- Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol : 4069–4075, 2005. - PubMed
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet : 319–323, 1967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources