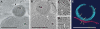Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell-cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography - PubMed (original) (raw)
Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell-cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography
Benjamin Junglas et al. Arch Microbiol. 2008 Sep.
Abstract
Ultrastructure and intercellular interaction of Ignicoccus hospitalis and Nanoarchaeum equitans were investigated using two different electron microscopy approaches, by three-dimensional reconstructions from serial sections, and by electron cryotomography. Serial sections were assembled into 3D reconstructions, for visualizing the unusual complexity of I. hospitalis, its huge periplasmic space, the vesiculating cytoplasmic membrane, and the outer membrane. The cytoplasm contains fibres which are reminiscent to a cytoskeleton. Cell division in I. hospitalis is complex, and different to that in Euryarchaeota or Bacteria. An irregular invagination of the cytoplasmic membrane is followed by separation of the two cytoplasms. Simultaneous constriction of cytoplasmic plus outer membrane is not observed. Cells of N. equitans show a classical mode of cell division, by constriction in the mid-plane. Their cytoplasm exhibits two types of fibres, elongated and ring-shaped. Electron micrographs of contact sites between I. hospitalis and N. equitans exhibit two modes of interaction. One is indirect and mediated by thin fibres; in other cells the two cell surfaces are in direct contact. The two membranes of I. hospitalis cells are frequently seen in direct contact, possibly a prerequisite for transporting metabolites or substrates from the cytoplasm of one cell to the other. Rarely, a transport based on cargo vesicles is observed between I. hospitalis and N. equitans.
Figures
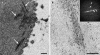
Fig. 1
Filaments in the cytoplasm of I. hospitalis cells, observed in transmission electron micrographs of ultrathin sections. Following cultivation in cellulose capillaries, cells were cryo-immobilized, freeze-substituted, and embedded in Epon. For a cells were freeze-substituted in AOUH; for b in EGFU. a Cytoplasmic filaments are marked by white arrows. b Cytoplasmic filaments composed of protofilaments, with regular arrangement of subunits. Inset in d Fourier spectrum of the longitudinal fibre. White arrows point to periodicities at (15 nm)−1 and (7.5 nm)−1 on the longitudinal axis, and at (20 nm)−1 and (10 nm)−1 on the transverse axis. Bars 200 nm
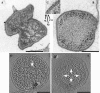
Fig. 2
Stages of cell division in I. hospitalis, observed in transmission electron micrographs of ultrathin sections. In all three cells, the two cytoplasms are almost or fully separated, but yet included in one outer membrane. a Cell in the early stage of division; the gap between the two cytoplasms is shown enlarged in d. b and c Cells in a later stage of division. Boxed area in b is shown enlarged in Fig. 3e. Cells were prepared as described in “Materials and methods”, with freeze-substitution in AOUH. Bars 1 μm (a–c), 200 nm (d)
Fig. 3
Outer membrane structure in I. hospitalis, observed in transmission electron micrographs of ultrathin sections, prepared as described in “Material and methods”; freeze-substitution was in AOUH. Three parts of the cell shown in c are enlarged in a, b, and d; the arrows point to the outer membrane, shown as asymmetric membrane (a), as symmetric membrane (b), and as split membrane (d). Outer membrane and cytoplasmic membrane in close and constant vicinity (Fig. 2b) are shown enlarged in e. Outer and cytoplasmic membrane are shown almost fused in f. Cy cytoplasm, CM cytoplasmic membrane, Pp periplasm, OM outer membrane. Bars 1 μm (c), 200 nm (a, b, d–f)
Fig. 4
3D reconstruction of half a cell of I. hospitalis on the basis of 28 serial ultrathin sections. Sample was prepared as described in “Materials and methods”. a–d Four selected equidistant sections of the whole data set. e 3D reconstruction of the data set, generated in AMIRA®, based on all ultrathin sections. Volumes of the cytoplasm and the periplasm are given in the text. White arrows point to periplasmic vesicles. Purple/rose cytoplasmic membrane. Pale yellow outer membrane. Bars 1 μm
Fig. 5
3D reconstruction of two-third of a cell of I. hospitalis on the basis of 23 serial ultrathin sections. Sample was prepared as described in “Materials and methods”. a–d Four selected sections of the whole data set. e, f 3D reconstruction, generated in AMIRA after stripping away the outer membrane, in order to visualize the periplasmic vesicles and the cytoplasmic membrane. e Side view; f top view. Orange surface area of the cytoplasmic membrane forming/releasing vesicles; yellow surface area of the smooth part of the cytoplasmic membrane, which is in close vicinity with the outer membrane. Horizontal white lines in the model in e indicate the approximate level of the sections shown in a–d, respectively. White arrows (in b, c, e, f) point to periplasmic vesicles; black arrows (in b, d) point to membrane vesicles in the cytoplasm. Bars 1 μm
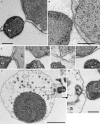
Fig. 6
Ultrastructure of N. equitans, incl. cell division, as seen by transmission electron microscopy of sections (a–c) and by electron cryotomography (d, e). For a–c cells were prepared for sections as described in “Materials and methods”; for a cells were freeze-substituted in AOUH; for b and c in EGFU. SL S-layer, Pp periplasmic space, CM cytoplasmic membrane. d, e XY slices from a tomogram of an N. equitans cell, obtained by ECT. White arrows point to longitudinal cytoplasmic filaments (d) and to ring-shaped filaments (e). Bars in a, b, d, e 200 nm. c Is part of b, and further enlarged by a factor of two
Fig. 7
Contact sites of I. hospitalis with cells of N. equitans in transmission electron micrographs of ultrathin sections. Samples were prepared as described in “Materials and methods”, and freeze-substituted in AOUH (a, c–h) and in EGFU (b). aI. hospitalis (Ih) and N. equitans (Ne) connected by fibrous material. b Outer membrane of I. hospitalis and the cell surface of N. equitans in direct contact, and, in addition, both membranes of I. hospitalis in close contact. c–f Four examples of the variation of contact sites; c direct contact of the I. hospitalis outer membrane with the cell surface of N. equitans. d, e Fibrous material in the gap between the two cells. f In this section plane, the contact between the two cells is not visible; the two I. hospitalis membranes are in direct contact. g A different type of contact site between I. hospitalis (Ih) and N. equitans (Ne), interconnected by fibrous material. Here, the periplasmic space in I. hospitalis is large and contains vesicles. Note the elongation and central indentation of the N. equitans cell, indicating an early phase of division. Bars 1 μm (g) and 200 nm (a, also for c–f, b, h)
Fig. 8
3D reconstruction of I. hospitalis and N. equitans, based on data obtained by electron cryotomography. a, b XY slices through the tomogram (effectively virtual sections, perpendicular to the electron optical axis) in different levels, obtained by 3D reconstruction of both cells. Ih I. hospitalis cell, Ne N. equitans cell. c, d Twofold enlarged parts of b. White arrows in b, c and d point to fibres between the cells. e Visualization of both cells after segmentation, using A
mira
. Red surface of the I. hospitalis cell; blue outer rim of the N. equitans cell; yellow fibres interconnecting both cells. Bars 500 nm
Similar articles
- Nanoarchaeum equitans and Ignicoccus hospitalis: new insights into a unique, intimate association of two archaea.
Jahn U, Gallenberger M, Paper W, Junglas B, Eisenreich W, Stetter KO, Rachel R, Huber H. Jahn U, et al. J Bacteriol. 2008 Mar;190(5):1743-50. doi: 10.1128/JB.01731-07. Epub 2007 Dec 28. J Bacteriol. 2008. PMID: 18165302 Free PMC article. - The unusual cell biology of the hyperthermophilic Crenarchaeon Ignicoccus hospitalis.
Huber H, Küper U, Daxer S, Rachel R. Huber H, et al. Antonie Van Leeuwenhoek. 2012 Aug;102(2):203-19. doi: 10.1007/s10482-012-9748-5. Epub 2012 Jun 1. Antonie Van Leeuwenhoek. 2012. PMID: 22653377 Review. - Life on the edge: functional genomic response of Ignicoccus hospitalis to the presence of Nanoarchaeum equitans.
Giannone RJ, Wurch LL, Heimerl T, Martin S, Yang Z, Huber H, Rachel R, Hettich RL, Podar M. Giannone RJ, et al. ISME J. 2015 Jan;9(1):101-14. doi: 10.1038/ismej.2014.112. Epub 2014 Jul 11. ISME J. 2015. PMID: 25012904 Free PMC article. - Proteomic characterization of cellular and molecular processes that enable the Nanoarchaeum equitans--Ignicoccus hospitalis relationship.
Giannone RJ, Huber H, Karpinets T, Heimerl T, Küper U, Rachel R, Keller M, Hettich RL, Podar M. Giannone RJ, et al. PLoS One. 2011;6(8):e22942. doi: 10.1371/journal.pone.0022942. Epub 2011 Aug 3. PLoS One. 2011. PMID: 21826220 Free PMC article. - Happy together: genomic insights into the unique Nanoarchaeum/Ignicoccus association.
Forterre P, Gribaldo S, Brochier-Armanet C. Forterre P, et al. J Biol. 2009;8(1):7. doi: 10.1186/jbiol110. Epub 2009 Jan 23. J Biol. 2009. PMID: 19216728 Free PMC article. Review.
Cited by
- A Micrarchaeon Isolate Is Covered by a Proteinaceous S-Layer.
Gfrerer S, Winkler D, Novion Ducassou J, Couté Y, Rachel R, Gescher J. Gfrerer S, et al. Appl Environ Microbiol. 2022 Mar 8;88(5):e0155321. doi: 10.1128/AEM.01553-21. Epub 2022 Jan 12. Appl Environ Microbiol. 2022. PMID: 35020453 Free PMC article. - Single-cell genomics of co-sorted Nanoarchaeota suggests novel putative host associations and diversification of proteins involved in symbiosis.
Jarett JK, Nayfach S, Podar M, Inskeep W, Ivanova NN, Munson-McGee J, Schulz F, Young M, Jay ZJ, Beam JP, Kyrpides NC, Malmstrom RR, Stepanauskas R, Woyke T. Jarett JK, et al. Microbiome. 2018 Sep 17;6(1):161. doi: 10.1186/s40168-018-0539-8. Microbiome. 2018. PMID: 30223889 Free PMC article. - Inter-species interconnections in acid mine drainage microbial communities.
Comolli LR, Banfield JF. Comolli LR, et al. Front Microbiol. 2014 Jul 25;5:367. doi: 10.3389/fmicb.2014.00367. eCollection 2014. Front Microbiol. 2014. PMID: 25120533 Free PMC article. - AMP-forming acetyl coenzyme A synthetase in the outermost membrane of the hyperthermophilic crenarchaeon Ignicoccus hospitalis.
Mayer F, Küper U, Meyer C, Daxer S, Müller V, Rachel R, Huber H. Mayer F, et al. J Bacteriol. 2012 Mar;194(6):1572-81. doi: 10.1128/JB.06130-11. Epub 2012 Jan 13. J Bacteriol. 2012. PMID: 22247508 Free PMC article. - Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons.
Ganjam GK, Bolte K, Matschke LA, Neitemeier S, Dolga AM, Höllerhage M, Höglinger GU, Adamczyk A, Decher N, Oertel WH, Culmsee C. Ganjam GK, et al. Cell Death Dis. 2019 Nov 14;10(11):865. doi: 10.1038/s41419-019-2091-2. Cell Death Dis. 2019. PMID: 31727879 Free PMC article.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/sj.emboj.7600366', 'is_inner': False, 'url': 'https://doi.org/10.1038/sj.emboj.7600366'}, {'type': 'PMC', 'value': 'PMC517607', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC517607/'}, {'type': 'PubMed', 'value': '15318169', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15318169/'}\]}
- Al-Amoudi A, Chang J-J, Leforestier A, McDowall A, Salamin LM, Norlén LPO, Richter K, Sartori Blanc N, Studer D, Dubochet J (2004) Cryo-electron microscopy of vitreous sections. EMBO J 23:3583–3588 - PMC - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1365-2958.2006.05355.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1365-2958.2006.05355.x'}, {'type': 'PubMed', 'value': '16987173', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16987173/'}\]}
- Briegel A, Prabha Dias D, Li Z, Jensen RB, Frangakis AS, Jensen GJ (2006) Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol Microbiol 62:5–14 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1111/j.1365-2958.2006.05509.x', 'is_inner': False, 'url': 'https://doi.org/10.1111/j.1365-2958.2006.05509.x'}, {'type': 'PubMed', 'value': '17163971', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17163971/'}\]}
- Burghardt T, Näther DJ, Junglas B, Huber H, Rachel R (2007) The dominating outer membrane protein of the hyperthermophilic archaeum Ignicoccus hospitalis: a novel pore-forming complex. Mol Microbiol 63:166–176 - PubMed
- Burghardt T, Saller M, Gürster S, Müller D, Meyer C, Jahn U, Hochmuth E, Deutzmann R, Siedler F, Babinger P, Wirth R, Huber H, Rachel R (2008) Insight into the proteome of the hyperthermophilic Crenarchaeon Ignicoccus hospitalis: the major cytosolic and membrane proteins. Arch Microbiol. doi:10.1007/s00203-008-0399-x - PMC - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1128/JVI.01564-06', 'is_inner': False, 'url': 'https://doi.org/10.1128/jvi.01564-06'}, {'type': 'PMC', 'value': 'PMC1865996', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1865996/'}, {'type': 'PubMed', 'value': '17192309', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17192309/'}\]}
- Buser C, Walther P, Mertens T, Michel D (2007) Cytomegalovirus primary envelopment occurs at large infoldings of the inner nuclear membrane. J Virol 81:3042–3048 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources