Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome - PubMed (original) (raw)
Randomized Controlled Trial
. 2008 Nov;23(11):2013-20.
doi: 10.1007/s00467-008-0899-6. Epub 2008 Jul 12.
Affiliations
- PMID: 18622632
- PMCID: PMC7462920
- DOI: 10.1007/s00467-008-0899-6
Randomized Controlled Trial
Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome
Eiske M Dorresteijn et al. Pediatr Nephrol. 2008 Nov.
Abstract
We performed a multi-centre randomized controlled trial to compare the efficacy of mycophenolate mofetil (MMF) to that of cyclosporine A (CsA) in treating children with frequently relapsing nephrotic syndrome and biopsy-proven minimal change disease. Of the 31 randomized initially selected patients, seven were excluded. The remaining 24 children received either MMF 1200 mg/m(2) per day (n = 12) or CsA 4-5 mg/kg per day (n = 12) during a 12-month period. Of the 12 patients in the MMF group, two discontinued the study medication. Evaluation of the changes from the baseline glomerular filtration rate showed an overall significant difference in favour of MMF over the treatment period (p = 0.03). Seven of the 12 patients in the MMF group and 11 of the 12 patients in the CsA group remained in complete remission during the entire study period. Relapse rate in the MMF group was 0.83/year compared to 0.08/year in the CsA group (p = 0.08). None of the patients reported diarrhea. Pharmacokinetic profiles of mycophenolic acid were performed in seven patients. The patient with the lowest area under the curve had three relapses within 6 months. In children with frequently relapsing minimal change nephrotic syndrome, MMF has a favourable side effect profile compared to CsA; however, there is a tendency towards a higher relapse risk in patients treated with MMF.
Figures
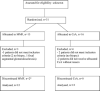
Fig. 1
Flow chart of the study comparing mycophenolate mofetil (MMF) and cyclosporine (CsA) for remission maintenance in nephrotic syndrome. Asterisk indicates frequent relapses. There was one request for withdrawal from the study
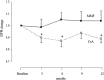
Fig. 2
Change in the glomerular filtration rate (GFR) from baseline for patients receiving mycophenolate mofetil (MMF, n = 12) and cyclosporine A (CsA, n = 12) medication. The change in the GFR is expressed as ratio compared to baseline. Data are given as ANOVA estimates with standard errors. *p < 0.05 compared to baseline
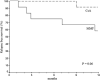
Fig. 3
Relapse-free survival curve for patients with mycophenolate mofetil (MMF, n = 12) and cyclosporine A (CsA, n = 12) medication. The p value is from the logrank test
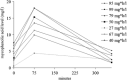
Fig. 4
Mycophenolic acid levels with area under the curve. Mycophenolic acid levels were measured using an immunoassay (filled symbols) or by high-performance liquid chromatography–ultraviolet test (open symbols). Unbroken line no relapses, broken line three relapses in 6 months
Similar articles
- Long-term outcome of Japanese children with complicated minimal change nephrotic syndrome treated with mycophenolate mofetil after cyclosporine.
Fujinaga S, Hirano D, Nishino T, Umeda C, Watanabe Y, Nakagawa M. Fujinaga S, et al. Pediatr Nephrol. 2019 Nov;34(11):2417-2421. doi: 10.1007/s00467-019-04339-y. Epub 2019 Aug 21. Pediatr Nephrol. 2019. PMID: 31435725 - Cyclosporine versus mycophenolate mofetil for maintenance of remission of steroid-dependent nephrotic syndrome after a single infusion of rituximab.
Fujinaga S, Someya T, Watanabe T, Ito A, Ohtomo Y, Shimizu T, Kaneko K. Fujinaga S, et al. Eur J Pediatr. 2013 Apr;172(4):513-8. doi: 10.1007/s00431-012-1913-3. Epub 2012 Dec 28. Eur J Pediatr. 2013. PMID: 23271494 - Sequential maintenance therapy with cyclosporin A and mycophenolate mofetil for sustained remission of childhood steroid-resistant nephrotic syndrome.
Gellermann J, Ehrich JH, Querfeld U. Gellermann J, et al. Nephrol Dial Transplant. 2012 May;27(5):1970-8. doi: 10.1093/ndt/gfr572. Epub 2011 Oct 4. Nephrol Dial Transplant. 2012. PMID: 21976740 - The treatment of relapse in adults with minimal change nephrotic syndrome: myths and facts.
Shigidi MM. Shigidi MM. Saudi J Kidney Dis Transpl. 2011 Jan;22(1):10-7. Saudi J Kidney Dis Transpl. 2011. PMID: 21196608 Review. - Can we eliminate both calcineurin inhibitors and steroids?
Lebranchu Y. Lebranchu Y. Transplant Proc. 2010 Nov;42(9 Suppl):S25-8. doi: 10.1016/j.transproceed.2010.07.002. Transplant Proc. 2010. PMID: 21095446 Review.
Cited by
- Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children.
Larkins NG, Hahn D, Liu ID, Willis NS, Craig JC, Hodson EM. Larkins NG, et al. Cochrane Database Syst Rev. 2024 Nov 8;11(11):CD002290. doi: 10.1002/14651858.CD002290.pub6. Cochrane Database Syst Rev. 2024. PMID: 39513526 Review. - Immunosuppressive agents for frequently relapsing/steroid-dependent nephrotic syndrome in children: a systematic review and network meta-analysis.
Zhu Y, Chen J, Zhang Y, Wang X, Wang J. Zhu Y, et al. Front Immunol. 2024 Feb 23;15:1310032. doi: 10.3389/fimmu.2024.1310032. eCollection 2024. Front Immunol. 2024. PMID: 38464533 Free PMC article. - Therapeutic drug monitoring in childhood idiopathic nephrotic syndrome: a state of the art review.
Lai FF, Chan EY, Tullus K, Ma AL. Lai FF, et al. Pediatr Nephrol. 2024 Jan;39(1):85-103. doi: 10.1007/s00467-023-05974-2. Epub 2023 May 6. Pediatr Nephrol. 2024. PMID: 37147510 Review. - Second and Third Generational Advances in Therapies of the Immune-Mediated Kidney Diseases in Children and Adolescents.
Grenda R, Obrycki Ł. Grenda R, et al. Children (Basel). 2022 Apr 11;9(4):536. doi: 10.3390/children9040536. Children (Basel). 2022. PMID: 35455580 Free PMC article. Review. - Mycophenolate Mofetil in the Treatment of Steroid-Dependent or Frequently Relapsing Nephrotic Syndrome in Children: A Meta-Analysis.
Xiang X, Qiu SY, Wang M. Xiang X, et al. Front Pediatr. 2021 Jun 15;9:671434. doi: 10.3389/fped.2021.671434. eCollection 2021. Front Pediatr. 2021. PMID: 34211944 Free PMC article.
References
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8:769–776. - PubMed
- Durkan A, Hodson EM, Willis NS, Craig JC (2005) Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev: CD002290 - PubMed
- International Study of Kidney Disease in Children Prospective controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Lancet. 1974;2:423–427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources